Abstract
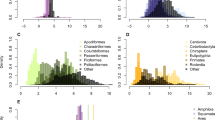
Measurements of variation are of great importance for studying the stability of pathological phenomena and processes. For the biology of aging, it is very important not only to determine average mortality, but also to study its stability in time and the size of fluctuations that are indicated by the variation coefficient of lifespan (CVLS). It is believed that a relatively small (∼20%) value of CVLS in humans, comparable to the coefficients of variation of other events programmed in ontogenesis (for example, menarche and menopause), indicates a relatively rigid determinism (N. S. Gavrilova et al. (2012) Biochemistry (Moscow), 77, 754-760). To assess the prevalence of this phenomenon, we studied the magnitude of CVLS, as well as the coefficients of skewness and kurtosis in diverse representatives of the animal kingdom using data provided by the Institute for Demographic Research (O. R. Jones et al. (2014) Nature, 505, 169-173). We found that, unlike humans and laboratory animals, in most examined species the values of CVLS are rather high, indicating heterogeneity of the lifespan in the cohorts studied. This is probably due to the large influence of background mortality, as well as the non-monotonicity of total mortality in the wild, especially at the earliest ages. One way to account for this influence is to “truncate” the data (removing the earliest and latest ages from consideration). To reveal the effect of this procedure, we proposed a new indicator, the stability coefficient of mortality dynamics, which indicates how quickly CVLS is reduced to values that characterize a relatively homogeneous population (33%) when the data are “truncated”. Such indicators facilitate the use of the parameters of survival curves for analysis of the effects of geroprotectors, lifestyle, and other factors on lifespan, and for the quantification of relative contributions of genetic and environmental factors to the dynamics of aging in human and animal populations, including those living in the wild.
Similar content being viewed by others
Abbreviations
- CV:
-
coefficient of variation
- LS:
-
lifespan
References
Gavrilova, N. S., Gavrilov, L. A., Severin, F. F., and Skulachev, V. P. (2012) Testing predictions of the programmed and stochastic theories of aging: comparison of variation in age at death, menopause, and sexual maturation, Biochemistry (Moscow), 77, 754–760.
Jones, O. R., Scheuerlein, A., Salguero-Gomez, R., Camarda, C. G., Schaible, R., Casper, B. B., Dahlgren, J. P., Ehrlen, J., Garcia, M. B., Menges, E. S., Quintana-Ascencio, P. F., Caswell, H., Baudisch, A., and Vaupel, J. W. (2014) Diversity of ageing across the tree of life, Nature, 505, 169–173.
Vaupel, J. W., Carey, J. R., Christensen, K., Johnson, T. E., Yashin, A. I., Holm, N. V., Iachine, I. A., Kannisto, V., Khazaeli, A. A., Liedo, P., Longo, V. D., Zeng, Y., Manton, K. G., and Curtsinger, J. W. (1998) Biodemographic trajectories of longevity, Science, 280, 855–860.
Baudisch, A. (2008) Inevitable Aging? Contributions to Evolutionary-Demographic Theory, Springer-Verlag, Berlin-Heidelberg.
Skulachev, M. V., and Skulachev, V. P. (2014) New data on programmed aging–slow phenoptosis, Biochemistry (Moscow), 79, 977–993.
Markov, A. V. (2012) Can kin selection facilitate the evolution of the genetic program of senescence? Biochemistry (Moscow), 77, 733–741.
Hyams, Y., Paz, G., Rabinowitz, C., and Rinkevich, B. (2017) Insights into the unique torpor of Botrylloides leachii, a colonial urochordate, Dev. Biol., 428, 101–117.
Fisher, R. A. (1930) The Genetical Theory of Natural Selection, Clarendon Press, Oxford.
Medawar, P. B. (1952) An Unsolved Problem of Biology, H. K. Lewis, London.
Hamilton, W. D. (1966) The moulding of senescence by natural selection, J. Theor. Biol., 12, 12–45.
Nusbaum, N. J. (1996) What good is it to get old? Med. Hypotheses, 47, 77–79.
Brent, L. J., Franks, D. W., Foster, E. A., Balcomb, K. C., Cant, M. A., and Croft, D. P. (2015) Ecological knowledge, leadership, and the evolution of menopause in killer whales, Curr. Biol., 25, 746–750.
Kirkwood, T. B. L. (2010) Systems biology of ageing and longevity, Phil. Trans. R. Soc. B., 366, 64–70.
Williams, G. C. (1957) Pleiotropy, natural selection and the evolution of senescence, Evolution, 11, 398–411.
Vijg, J., and Suh, Y. (2005) Genetics of longevity and aging, Annu. Rev. Med., 56, 193–212.
Campisi, J. (2005) Aging, tumor suppression and cancer: high wire-act! Mech. Ageing Dev., 126, 51–58.
Liu, J. J., Prescott, J., Giovannucci, E., Hankinson, S. E., Rosner, B., Han, J., and De Vivo, I. (2013) Plasma vitamin D biomarkers and leukocyte telomere length, Am. J. Epidemiol., 177, 1411–1417.
Zhu, Y., Tchkonia, T., Pirtskhalava, T., Gower, A. C., Ding, H., Giorgadze, N., Palmer, A. K., Ikeno, Y., Hubbard, G. B., Lenburg, M., O’Hara, S. P., LaRusso, N. F., Miller, J. D., Roos, C. M., Verzosa, G. C., LeBrasseur, N. K., Wren, J. D., Farr, J. N., Khosla, S., Stout, M. B., McGowan, S. J., Fuhrmann-Stroissnigg, H., Gurkar, A. U., Zhao, J., Colangelo, D., Dorronsoro, A., Ling, Y. Y., Barghouthy, A. S., Navarro, D. C., Sano, T., Robbins, P. D., Niedernhofer, L. J., and Kirkland, J. L. (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs, Aging Cell, 14, 644–658.
Skulachev, V. P. (1997) Aging is a specific biological function rather than the result of a disorder in complex living systems: biochemical evidence in support of Weismann’s hypothesis, Biochemistry (Moscow), 62, 1191–1195.
Skulachev, V. P. (1999) Phenoptosis: programmed death of an organism? Biochemistry (Moscow), 64, 1418–1426.
Weismann, A. (1889) Essays upon Heredity and Kindred Biological Problems, Oxford, Clarendon Press.
Guarente, L., and Kenyon, C. (2000) Genetic pathways that regulate ageing in model organisms, Nature, 408, 255–262.
Longo, V. D., Mitteldorf, J., and Skulachev, V. P. (2005) Programmed and altruistic ageing, Nature Rev. Genet., 6, 866–872.
Fushan, A. A., Turanov, A. A., Lee, S. G., Kim, E. B., Lobanov, A. V., Yim, S. H., Buffenstein, R., Lee, S. R., Chang, K. T., Rhee, H., Kim, J. S., Yang, K. S., and Gladyshev, V. N. (2015) Gene expression defines natural changes in mammalian lifespan, Aging Cell, 14, 352–365.
Ma, S., and Gladyshev, V. N. (2017) Molecular signatures of longevity: insights from cross-species comparative studies, Semin. Cell. Dev. Biol., pii: S1084–9521.
Ashapkin, V. V., Kutueva, L. I., and Vanyushin, B. F. (2015) Aging epigenetics: accumulation of errors or realization of a specific program? Biochemistry (Moscow), 80, 1406–1417.
Stegeman, R., and Weake, V. M. (2017) Transcriptional signatures of aging, J. Mol. Biol., 429, 2427–2437.
Austad, S. N. (1993) Retarded senescence in an insular population of opossums, J. Zool. (Lond.), 229, 695–708.
Skulachev, M. V., Severin, F. F., and Skulachev, V. P. (2015) Aging as an evolvability-increasing program which can be switched off by organism to mobilize additional resources for survival, Curr. Aging Sci., 8, 95–109.
Hughes, B. G., and Hekimi, S. (2017) Many possible maximum lifespan trajectories, Nature, 546, 8–9.
Gompertz, B. (1825) On the nature of the function expressive of the law of human mortality and on a new mode of determining life contingencies, Philos. Trans. R. Soc. L. A., 115, 513–585.
Deevey, E. S. (1947) Life tables for natural populations of animals, Q. Rev. Biol., 22, 283–314.
Finch, C. E. (1990) Longevity, Senescence and the Genome, University Chicago Press, Chicago.
Gavrilov, L. A., and Gavrilova, N. S. (1991) The Biology of Life Span: A Quantitative Approach, Harwood Academic Publisher, N. Y.
Khalyavkin, A. V. (2001) Influence of environment on the mortality pattern of potentially non-senescent organisms. General approach and comparison with real populations, Adv. Gerontol., 7, 46–49.
Ricklefs, R. E. (2010) Life-history connections to rates of aging in terrestrial vertebrates, Proc. Natl. Acad. Sci. USA, 107, 10314–10319.
Stroustrup, N., Anthony, W. E., Nash, Z. M., Gowda, V., Gomez, A., Lopez-Moyado, I. F., Apfeld, J., and Fontana, W. (2016) The temporal scaling of Caenorhabditis elegans ageing, Nature, 530, 103–107.
Warner, D. A., Miller, D. A., Bronikowski, A. M., and Janzen, F. J. (2016) Decades of field data reveal that turtles senesce in the wild, Proc. Natl. Acad. Sci. USA, 113, 6502–6507.
Mitnitski, A., Howlett, S. E., and Rockwood, K. (2017) Heterogeneity of human aging and its assessment, J. Gerontol. A Biol. Sci. Med. Sci., 72, 877–884.
Baudisch, A., and Vaupel, J. (2010) Senescence vs. sustenance: evolutionary-demographic models of aging, Demogr. Res., 23, 655–668.
Mitnitski, A., Song, X., and Rockwood, K. (2013) Assessing biological aging: the origin of deficit accumulation, Biogerontology, 14, 709–717.
Shilovsky, G. A., Putyatina, T. S., Markov, A. V., and Skulachev, V. P. (2015) Contribution of quantitative methods of estimating mortality dynamics to explaining mechanisms of aging, Biochemistry (Moscow), 80, 1547–1559.
Akif’ev, A. P., and Potapenko, A. I. (2001) Nuclear genetic material as an initial substrate of aging in animals, Russ. J. Genet., 37, 1213–1223.
Blagosklonny, M. V. (2007) Program-like aging and mitochondria: instead of random damage by free radicals, J. Cell. Biochem., 102, 1389–1399.
Goldsmith, T. C. (2008) Aging, evolvability, and the individual benefit requirement; medical implications of aging theory controversies, J. Theor. Biol., 252, 764–768.
Libertini, G. (2012) Phenoptosis, another specialized neologism, or the mark of a widespread revolution? Biochemistry (Moscow), 77, 795–798.
Khokhlov, A. N. (2010) Does aging need an own program or the existing development program is more than enough, Russ. J. Gen. Chem., 80, 1507–1513.
Shilovsky, G. A., Khokhlov, A. N., and Shram, S. I. (2013) The protein poly(ADP-ribosyl)ation system: its role in genome stability and lifespan determination, Biochemistry (Moscow), 78, 433–444.
Khalyavkin, A. V. (2013) Phenoptosis as genetically determined aging influenced by signals from the environment, Biochemistry (Moscow), 78, 1001–1005.
Skulachev, V. P., Holtze, S., Vyssokikh, M. Y., Bakeeva, L. E., Skulachev, M. V., Markov, A. V., Hildebrandt, T. B., and Sadovnichii, V. A. (2017) Neoteny, prolongation of youth: from naked mole rats to “naked apes” (humans), Physiol. Rev., 97, 699–720.
Timofeeff-Ressovsky, N. W. (1927) Studies on the phenotype manifestation of hereditary factors. I. On the phenotypic manifestation of the genovariation radius incompletus in Drosophila funebris, Genetics, 12, 128–198.
Comfort, A. (1979) The Biology of Senescence, Churchill Livingstone, Edinburgh-London.
Carey, J. R., and Judge, D. S. (2001) Odense Monographs on Population Aging, Ser. 8, Odense University Press, Odense, Denmark.
Bowles, J. T. (1998) The evolution of aging: a new approach to an old problem of biology, Med. Hypotheses, 51, 179–221.
Terres, J. (1980) The Audubon Society Encyclopedia of North American Birds, Knopf, New York.
Murphy, R., Berry, K., Edwards, T., Leviton, A., Lathrop, A., and Riedle, J. (2011) The dazed and confused identity of Agassiz’s land tortoise, Gopherus agassizii (Testudines: Testudinidae) with the description of a new species and its consequences for conservation, ZooKeys, 113, 39–71.
Miller, J. K. (2001) Escaping senescence: demographic data from the three-toed box turtle (Terrapene carolina triunguis), Exp. Gerontol., 36, 829–832.
Congdon, J. D., Nagle, R. D., Kinney, O. M., van Loben Sels, R. C., Quinter, T., and Tinkle, D. W. (2003) Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta), Exp. Gerontol., 38, 765–772.
Shilovsky, G. A., Putyatina, T. S., Lysenkov, S. N., Ashapkin, V. V., Luchkina, O. S., Markov, A. V., and Skulachev, V. P. (2016) Is it possible to prove the existence of an aging program by quantitative analysis of mortality dynamics? Biochemistry (Moscow), 81, 1461–1476.
Finch, C. E., and Tanzi, R. E. (1997) Genetics of aging, Science, 278, 407–411.
Cohen, A. A. (2017) Aging across the tree of life: the importance of a comparative perspective for the use of animal models in aging, Biochim. Biophys. Acta, pii: S0925–4439.
Buffenstein, R. (2005) The naked mole-rat: a new long-living model for human aging research, J. Gerontol. A Biol. Sci. Med. Sci., 60, 1369–1377.
Buffenstein, R. (2008) Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species, J. Comp. Physiol. B, 178, 439–445.
Gavrilov, L. A., Gavrilova, N. S., and Yaguzhinsky, L. S. (1978) The main directions of the aging and death of animals from the point of view of reliability theory, J. Gen. Biol., 39, 734–742.
Tarkhov, A. E., Menshikov, L. I., and Fedichev, P. O. (2017) Strehler–Mildvan correlation is a degenerate manifold of Gompertz fit, J. Theor. Biol., 416, 180–189.
Jones, O. R., Gaillard, J. M., Tuljapurkar, S., Alho, J. S., Armitage, K. B., Becker, P. H., Bize, P., Brommer, J., Charmantier, A., Charpentier, M., Clutton-Brock, T., Dobson, F. S., Festa-Bianchet, M., Gustafsson, L., Jensen, H., Jones, C. G., Lillandt, B. G., McCleery, R., Merilä, J., Neuhaus, P., Nicoll, M. A., Norris, K., Oli, M. K., Pemberton, J., Pietiäinen, H., Ringsby, T. H., Roulin, A., Saether, B. E., Setchell, J. M., Sheldon, B. C., Thompson, P. M., Weimerskirch, H., Jean Wickings, E., and Coulson, T. (2008) Senescence rates are determined by ranking on the fast-slow life-history continuum, Ecol. Lett., 11, 664–673.
Baudisch, A. (2011) The pace and shape of ageing, Methods Ecol. Evol., 2, 375–382.
Markov, A. V., Naimark, E. B., and Yakovleva, E. U. (2016) Temporal scaling of age-dependent mortality: dynamics of aging in Caenorhabditis elegans is easy to speed up or slow down, but its overall trajectory is stable, Biochemistry (Moscow), 81, 906–911.
Nussey, D. H., Froy, H., Lemaitre, J. F., Gaillard, J. M., and Austad, S. N. (2013) Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology, Ageing Res. Rev., 12, 214–225.
Lamb, M. J. (1977) Biology of Aging, John Wiley and Sons, New York.
Voituron, Y., De Fraipont, M., Issartel, J., Guillaume, O., and Clobert, J. (2011) Extreme lifespan of the human fish (Proteus anguinus): a challenge for ageing mechanisms, Biol. Lett., 7, 105–107.
Anisimov, V. N. (2008) Molecular and Physiological Mechanisms of Aging [in Russian], Nauka, St. Petersburg.
Myl’nikov, S. V. (2011) Towards the estimation of survival curves parameters and geroprotectors classification, Adv. Gerontol., 24, 563–569.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G. A. Shilovsky, T. S. Putyatina, V. V. Ashapkin, O. S. Luchkina, A. V. Markov, 2017, published in Biokhimiya, 2017, Vol. 82, No. 12, pp. 1842-1857.
Rights and permissions
About this article
Cite this article
Shilovsky, G.A., Putyatina, T.S., Ashapkin, V.V. et al. Coefficient of variation of lifespan across the tree of life: Is it a signature of programmed aging?. Biochemistry Moscow 82, 1480–1492 (2017). https://doi.org/10.1134/S0006297917120070
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917120070