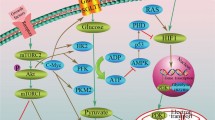
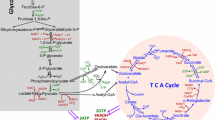
Abstract
Investigation of cancer cell metabolism has revealed variability of the metabolic profiles among different types of tumors. According to the most classical model of cancer bioenergetics, malignant cells primarily use glycolysis as the major metabolic pathway and produce large quantities of lactate with suppressed oxidative phosphorylation even in the presence of ample oxygen. This is referred to as aerobic glycolysis, or the Warburg effect. However, a growing number of recent studies provide evidence that not all cancer cells depend on glycolysis, and, moreover, oxidative phosphorylation is essential for tumorigenesis. Thus, it is necessary to consider distinctive patterns of cancer metabolism in each specific case. Chemoresistance of cancer cells is associated with decreased sensitivity to different types of antitumor agents. Stimulation of apoptosis is a major strategy for elimination of cancer cells, and therefore activation of mitochondrial functions with direct impact on mitochondria to destabilize them appears to be an important approach to the induction of cell death. Consequently, the design of combination therapies using acclaimed cytotoxic agents directed to induction of apoptosis and metabolic agents affecting cancer cell bioenergetics are prospective strategies for antineoplastic therapy.
Similar content being viewed by others
Abbreviations
- ANT:
-
adenine nucleotide translocase
- α-TOS:
-
a-tocopheryl succinate
- CypD:
-
cyclophilin D
- DCA:
-
dichloroacetate
- 2-DG:
-
2-deoxyglucose
- HIF:
-
hypoxia inducible factor
- HK:
-
hexokinase
- MOM(P):
-
mitochondrial outer membrane (permeabilization)
- MPT(P):
-
mitochondrial permeability transition (pore)
- OXPHOS:
-
oxidative phosphorylation
- ROS:
-
reactive oxygen species
- VDAC:
-
voltage-dependent anion channel.
References
Hanahan, D., and Weinberg, R. A. (2011) Hallmarks of cancer: the next generation, Cell, 144, 646–674.
Lunt, S. Y., and Heiden, M. V. (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation, Annu. Rev. Cell Dev. Biol., 27, 441–464.
Tennant, D. A., Duran, R. V., Boulahbel, H., and Gottlieb, E. (2009) Metabolic transformation in cancer, Carcinogenesis, 30, 1269–1280.
Funes, J. M., Quintero, M., Henderson, S., Martinez, D., Qureshi, U., Westwood, C., Clements, M. O., Bourboulia, D., Pedley, R. B., Moncada, S., and Boshoff, C. (2007) Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production, Proc. Natl. Acad. Sci. USA, 104, 6223–6228.
Moreno-Sanchez, R., Rodriguez-Enriquez, S., MarinHernandez, A., and Saavedra, E. (2007) Energy metabolism in tumor cells, FEBS J., 274, 1393–1418.
Rodriguez-Enriquez, S., Carreno-Fuentes, L., GallardoPerez, J. C., Saavedra, E., Quezada, H., Vega, A., MarinHernandez, A., Olin-Sandoval, V., Torres-Marquez, M. E., and Moreno-Sanchez, R. (2010) Oxidative phosphorylation is impaired by prolonged hypoxia in breast and possibly in cervix carcinoma, Int. J. Biochem. Cell Biol., 42, 1744–1751.
Barbosa, I. A., Machado, N. G., Skildum, A. J., Scott, P. M., and Oliveira, P. J. (2012) Mitochondrial remodeling in cancer metabolism and survival: potential for new therapies, Biochim. Biophys. Acta, 1826, 238–254.
Ralph, S. J., Rodriguez-Enriquez, S., Neuzil, J., and Moreno-Sanchez, R. (2010) Bioenergetic pathways in tumor mitochondria as targets for cancer therapy and the importance of the ROS-induced apoptotic trigger, Mol. Aspects Med., 31, 29–59.
Vaughn, A., and Deshmukh, M. (2008) Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c, Nat. Cell Biol., 10, 1477–1483.
Cairns, R. A., Harris, I. S., and Mak, T. W. (2011) Regulation of cancer cell metabolism, Nat. Rev. Cancer, 11, 85–95.
Gao, P., Tchernyshyov, I., Chang, T., and Lee, Y. (2009) cMyc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism, Nature, 458, 762–765.
Wise, D. R., Deberardinis, R. J., Mancuso, A., Sayed, N., Zhang, X., Pfeiffer, H. K., Nissim, I., Daikhin, E., Yudkoff, M., Mcmahon, S. B., and Thompson, C. B. (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction, PNAS, 105, 18782–18787.
Dang, C. V. (2010) Rethinking the Warburg effect with Myc micromanaging glutamine metabolism, Cancer Res., 70, 859–862.
Jose, C., Bellance, N., and Rossignol, R. (2011) Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim. Biophys. Acta, 1807, 552–561.
Mazurek, S., Michel, A., and Eigenbrodt, E. (1997) Effect of extracellular AMP on cell proliferation and metabolism of breast cancer cell lines with high and low glycolytic rates, J. Biol. Chem., 272, 4941–4952.
Rossignol, R., Gilkerson, R., and Aggeler, R. (2004) Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells, Cancer Res., 64, 985–993.
Moreadith, R., and Lehninger, A. (1984) Purification, kinetic behavior, and regulation of NAD(P)+ malic enzyme of tumor mitochondria, J. Biol. Chem., 259, 6222–6227.
Mandella, R., and Sauer, L. (1975) The mitochondrial malic enzymes. I. Submitochondrial localization and purification and properties of the NAD(P)+-dependent enzyme from adrenal cortex, J. Biol. Chem., 250, 5877–5884.
Brand, R. M., Lyons, R. H., and Midgley, A. R. (1994) Understanding the dynamics of cellular responsiveness to modifications of metabolic substrates in perifusion, J. Cell. Physiol., 160, 10–16.
Zu, X. L., and Guppy, M. (2004) Cancer metabolism: facts, fantasy, and fiction, Biochem. Biophys. Res. Commun., 313, 459–465.
Vander Heiden, M. G., Cantley, L. C., and Thompson, C. B. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation, Science, 324, 1029–1033.
Gorlach, A., and Acker, H. (1994) pO2and pH-gradients in multicellular spheroids and their relationship to cellular metabolism and radiation sensitivity of malignant human tumor cells, Biochim. Biophys. Acta, 1227, 105–112.
Sutherland, R. (1998) Tumor hypoxia and gene expression, Acta Oncol., 37, 567–574.
Vaupel, P., Kallinowski, F., and Okunieff, P. (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review, Cancer Res., 49, 6449–6465.
Matsumoto, A., Matsumoto, S., Sowers, A., Koscielniak, J., Trigg, N., Kuppusamy, P., Mitchell, J., Subramanian, S., Krishna, M., and Matsumoto, K. (2005) Absolute oxygen tension (pO2) in murine fatty and muscle tissue as determined by EPR, Magn. Reson. Med., 54, 1530–1535.
Schroeder, T., Yuan, H., Viglianti, B., Peltz, C., Asopa, S., Vujaskovic, Z., and Dewhirst, M. (2005) Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat, Cancer Res., 65, 5163–5171.
Mason, M. G., Nicholls, P., Wilson, M. T., and Cooper, C. E. (2006) Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase, Proc. Natl. Acad. Sci. USA, 103, 708–713.
Gnaiger, E., Lassnig, B., Kuznetsov, A., Riege, A. G., and Margreiter, R. (1998) Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase, J. Exp. Biol., 201, 1129–1139.
Pecina, P., Gnaiger, E., Zeman, J., Pronicka, E., and Houstek, T. (2004) Decreased affinity for oxygen of cytochromec oxidase in Leigh syndrome caused by SURF1 mutations, Am. J. Physiol. Cell Physiol., 287, C1384–C1388.
Matoba, S., Kang, J.-G., Patino, W. D., Wragg, A., Boehm, M., Gavrilova, O., Hurley, P. J., Bunz, F., and Hwang, P. M. (2006) P53 regulates mitochondrial respiration, Science, 312, 1650–1653.
Pollard, P. J., Wortham, N. C., and Tomlinson, I. P. M. (2003) The TCA cycle and tumorigenesis: the examples of fumarate hydratase and succinate dehydrogenase, Ann. Med., 35, 632–639.
Robey, I. F., Lien, A. D., Welsh, S. J., Baggett, B. K., and Gillies, R. J. (2005) Hypoxia-inducible factor-1α and the glycolytic phenotype in tumors, Neoplasia, 7, 324–330.
Kroemer, G. (2006) Mitochondria in cancer, Oncogene, 25, 4630–4632.
Yeunga, S. J., Pand, J., and Leec, M.-H. (2008) Roles of p53, Myc and HIF-1 in regulating glycolysis–the seventh hallmark of cancer, Cell. Mol. Life Sci., 65, 3981–3999.
Shaw, R. J. (2006) Glucose metabolism and cancer, Curr. Opin. Cell Biol., 18, 598–608.
Parlo, R., and Coleman, P. (1984) Enhanced rate of citrate export from cholesterol-rich hepatoma mitochondria. The truncated Krebs cycle and other metabolic ramifications of mitochondrial membrane cholesterol, J. Biol. Chem., 259, 9997–10003.
Briscoe, D., Fiskum, G., Holleran, A., and Kelleher, J. (1994) Acetoacetate metabolism in AS-30D hepatoma cells, Mol. Cell Biochem., 136, 131–137.
Dietzen, D., and Davis, E. (1993) Oxidation of pyruvate, malate, citrate, and cytosolic reducing equivalents by AS-30D hepatoma mitochondria, Arch. Biochem. Biophys., 305, 91–102.
Schmitt, S., Schulz, S., Schropp, E.-M., Eberhagen, C., Simmons, A., Beisker, W., Aichler, M., and Zischka, H. (2014) Why to compare absolute numbers of mitochondria, Mitochondrion, 19 (Pt. A), 113–123.
Pedersen, P. (1978) Tumor mitochondria and the bioenergetics of cancer cells, Prog. Exp. Tumor Res., 22, 190–274.
LaNoue, K., Hemington, J., Ohnishi, T., Morris, H., and Williamson, J. (1974) Defects in anion and electron transport in Morris hepatoma mitochondria, Horm. Cancer, 131–167.
Lichtor, T., and Dohrmann, G. (1987) Oxidative metabolism and glycolysis in benign brain tumors, Neurosurgery, 67, 336–340.
Melo, R., Stevan, F., Campello, A., Carnieri, E., and de Oliveira, M. (1998) Occurrence of the Crabtree effect in HeLa cells, Cell Biochem. Funct., 16, 99–105.
Sauer, L. (1977) On the mechanism of the Crabtree effect in mouse ascites tumor cells, J. Cell Physiol., 93, 313–316.
Sussman, I., Erecinska, M., and Wilson, D. (1980) Regulation of cellular energy metabolism: the Crabtree effect, Biochim. Biophys. Acta, 591, 209–223.
Seshagiri, P., and Bavister, B. (1991) Glucose and phosphate inhibit respiration and oxidative metabolism in cultured hamster eight-cell embryos: evidence for the “Crabtree effect”, Mol. Reprod. Dev., 30, 105–111.
Yang, X., Borg, L., and Eriksson, U. (1997) Altered metabolism and superoxide generation in neural tissue of rat embryos exposed to high glucose, Am. J. Physiol., E173–E180.
Rodriguez-Enriquez, S., Juarez, O., Rodriguez-Zavala, J. S., and Moreno-Sanchez, R. (2001) Multisite control of the Crabtree effect in ascites hepatoma cells, Eur. J. Biochem., 268, 2512–2519.
Covian, R., and Moreno-Sanchez, R. (2001) Role of protonatable groups of bovine heart bc1 complex in ubiquinol binding and oxidation, Eur. J. Biochem., 268, 5783–5790.
Tsujimoto, Y., Ikegaki, N., and Croce, C. M. (1987) Characterization of the protein product of bcl-2, the gene involved in human follicular lymphoma, Oncogene, 2, 3–7.
Belmar, J., and Fesik, S. W. (2014) Small molecule Mcl-1 inhibitors for the treatment of cancer, Pharmacol. Ther., 145, 76–84.
Dutta, S., Gulla, S., Chen, T. S., Fire, E., Grant, R. A., and Keating, A. E. (2010) Determinants of BH3 binding specificity for Mcl-1 versus Bcl-xL, J. Mol. Biol., 398, 747–762.
Moldoveanu, T., Follis, A. V., Kriwacki, R. W., and Green, D. R. (2014) Many players in BCL-2 family affairs, Trends Biochem. Sci., 39, 101–111.
Chen, L., Willis, S., Wei, A., Smith, B., Fletcher, J., Hinds, M., Colman, P., Day, C., Adams, J., and Huang, D. (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function, Mol. Cell, 17, 393–403.
Biasutto, L., Dong, L.-F., Zoratti, M., and Neuzil, J. (2010) Mitochondrially targeted anti-cancer agents, Mitochondrion, 10, 670–681.
Gogvadze, V., Orrenius, S., and Zhivotovsky, B. (2008) Mitochondria in cancer cells: what is so special about them? Trends Cell Biol., 18, 165–173.
Hartman, M., and Czyz, M. (2012) Pro-apoptotic activity of BH3-only proteins and BH3 mimetics: from theory to potential cancer therapy, Anticancer Agents Med. Chem., 12, 966–981.
Gogvadze, V., Robertson, J. D., Zhivotovsky, B., and Orrenius, S. (2001) Cytochrome c release occurs via Ca2+dependent and Ca2+-independent mechanisms that are regulated by Bax, J. Biol. Chem., 276, 19066–19071.
Armstrong, J. S. (2006) The role of the mitochondrial permeability transition in cell death, Mitochondrion, 6, 225–234.
Narita, M., Shimizu, S., Ito, T., Chittenden, T., Lutz, R. J., Matsuda, H., and Tsujimoto, Y. (1998) Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria, Proc. Natl. Acad. Sci. USA, 95, 14681–14686.
Brenner, C., Cadiou, H., Vieira, H. L., Zamzami, N., Marzo, I., Xie, Z., Leber, B., Andrews, D., Duclohier, H., Reed, J. C., and Kroemer, G. (2000) Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator, Oncogene, 19, 329–336.
Crompton, M., Barksby, E., Johnson, N., and Capano, M. (2002) Mitochondrial intermembrane junctional complexes and their involvement in cell death, Biochimie, 84, 143–152.
Marzo, I., Brenner, C., Zamzami, N., Jurgensmeier, J. M., Susin, S. A., Vieira, H. L., Prevost, M. C., Xie, Z., Matsuyama, S., Reed, J. C., and Kroemer, G. (1998) Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis, Science, 281, 2027–2031.
Rostovtseva, T. K., Antonsson, B., Suzuki, M., Youle, R. J., Colombini, M., and Bezrukov, S. M. (2004) Bid, but not Bax, regulates VDAC channels, J. Biol. Chem., 279, 13575–13583.
Green, D. R., and Kroemer, G. (2004) The pathophysiology of mitochondrial cell death, Science, 305, 626–629.
Arora, K. K., and Pedersen, P. L. (1988) Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP, J. Biol. Chem., 263, 17422–17428.
Vander Heiden, M. G., Li, X. X., Gottleib, E., Hill, R. B., Thompson, C. B., and Colombini, M. (2001) Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane, J. Biol. Chem., 276, 19414–19419.
Tan, W., and Colombini, M. (2007) VDAC closure increases calcium ion flux, Biochim. Biophys. Acta, 29, 2510–2515.
Shulga, N. (2009) Hexokinase II detachment from the mitochondria potentiates cisplatin induced cytotoxicity through a caspase-2 dependent mechanism, Cell Cycle, 8, 3355–3364.
Mathupala, S., Ko, Y., and Pedersen, P. (2012) Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria, Oncogene, 25, 4777–4786.
Pastorino, J., and Hoek, J. (2008) Regulation of hexokinase binding to VDAC, J. Bioenerg. Biomembr., 40, 171–182.
Schindler, A., and Foley, E. (2013) Hexokinase 1 blocks apoptotic signals at the mitochondria, Cell. Signal., 25, 2685–2692.
Robey, R. B., and Hay, N. (2006) Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt, Oncogene, 25, 4683–4696.
Shinohara, Y., Ishida, T., Hino, M., Yamazaki, N., Baba, Y., and Terada, H. (2000) Characterization of porin isoforms expressed in tumor cells, Eur. J. Biochem., 267, 6067–6073.
Kennedy, S. G., Kandel, E. S., Cross, T. K., and Hay, N. (1999) Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria, Mol. Cell. Biol., 19, 5800–5810.
Yamaguchi, A., Tamatani, M., Matsuzaki, H., Namikawa, K., Kiyama, H., Vitek, M. P., Mitsuda, N., and Tohyama, M. (2001) Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53, J. Biol. Chem., 276, 5256–5264.
Del Peso, L., Gonzalez-Garcia, M., Page, C., Herrera, R., and Nunez, G. (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt, Science, 278, 687–699.
Franke, T. F., Hornik, C. P., Segev, L., Shostak, G. A., and Sugimoto, C. (2003) PI3K/Akt and apoptosis: size matters, Oncogene, 22, 8983–8998.
Gogvadze, V., Zhivotovsky, B., and Orrenius, S. (2010) The Warburg effect and mitochondrial stability in cancer cells, Mol. Aspects Med., 31, 60–74.
Majewski, N., Nogueira, V., Bhaskar, P., Coy, P. E., Skeen, J. E., Gottlob, K., Chandel, N. S., Thompson, C. B., Robey, R. B., and Hay, N. (2004) Hexokinase–mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak, Mol. Cell, 16, 819–830.
Mookherjee, P., and Quintanilla, R. (2007) Mitochondrialtargeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death, J. Cell Biochem., 102, 196–210.
Weinberg, S. E., and Chandel, N. S. (2015) Targeting mitochondria metabolism for cancer therapy, Nat. Publ. Gr., 11, 9–15.
Neuzil, J., Dong, L. F., Rohlena, J., Truksa, J., and Ralph, S. J. (2013) Classification of mitocans, anti-cancer drugs acting on mitochondria, Mitochondrion, 13, 199–208.
Lea, M. A., Qureshi, M. S., Buxhoeveden, M., Gengel, N., Kleinschmit, J., and DesBordes, C. (2013) Regulation of the proliferation of colon cancer cells by compounds that affect glycolysis, including 3-bromopyruvate, 2-deoxyglucose and biguanides, Anticancer Res., 33, 401–407.
Loar, P., Wahl, H., Kshirsagar, M., Gossner, G., Griffith, K., and Liu, J. R. (2010) Inhibition of glycolysis enhances cisplatin-induced apoptosis in ovarian cancer cells, Am. J. Obstet. Gynecol., 202, 1–8.
Maher, J. C., Krishan, A., and Lampidis, T. J. (2004) Greater cell cycle inhibition and cytotoxicity induced by 2deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions, Cancer Chemother. Pharmacol., 53, 116–122.
Sullivan, E. J., Kurtoglu, M., Brenneman, R., Liu, H., and Lampidis, T. J. (2014) Targeting cisplatin-resistant human tumor cells with metabolic inhibitors, Cancer Chemother. Pharmacol., 73, 417–427.
Birsoy, K., Wang, T., Possemato, R., Yilmaz, O., Koch, C., Chen, W., Hutchins, A., Gultekin, Y., Peterson, T., Carette, J., Brummelkamp, T., Clish, C., and Sabatini, D. M. (2012) MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors, Changes, 29, 997–1003.
Urakami, K., Zangiacomi, V., Yamaguchi, K., and Kusuhara, M. (2013) Impact of 2-deoxy-D-glucose on the target metabolome profile of a human endometrial cancer cell line, Biomed. Res., 34, 221–229.
Xu, R., Pelicano, H., and Zhou, Y. (2005) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia, 65, 613–621.
Ralph, S. J., Low, P., Dong, L., Lawen, A., and Neuzil, J. (2006) Mitocans: mitochondrial targeted anti-cancer drugs as improved therapies and related patent documents, Recent Pat. Anticancer Drug Discov., 1, 327–346.
Zu, X. L., and Guppy, M. (2004) Cancer metabolism: facts, fantasy, and fiction, Biochem. Biophys. Res. Commun., 313, 459–465.
Dang, C. V., and Semenza, G. L. (1999) Oncogenic alterations of metabolism, Trends Biochem. Sci., 24, 68–72.
Griguer, C. E., Oliva, C. R., and Gillespie, G. Y. (2005) Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines, J. Neurooncol., 74, 123–133.
Mathupala, S. P., Ko, Y. H., and Pedersen, P. L. (2010) The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies, Biochim. Biophys. Acta–Bioenergetics, 1797, 1225–1230.
Solaini, G., Sgarbi, G., and Baracca, A. (2011) Oxidative phosphorylation in cancer cells, Biochim. Biophys. Acta, 1807, 534–542.
Reitzer, L., Wice, B., and Kennell, D. (1979) Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells, J. Biol. Chem., 254, 2669–2676.
Fuchs, B. C., and Bode, B. P. (2006) Stressing out over survival: glutamine as an apoptotic modulator, J. Surg. Res., 131, 26–40.
Yuneva, M., Zamboni, N., Oefner, P., Sachidanandam, R., and Lazebnik, Y. (2007) Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells, J. Cell Biol., 178, 93–105.
Ladurner, A. G. (2006) Rheostat control of gene expression by metabolites, Mol. Cell, 24, 1–11.
Jonas, E. A., Hickman, J. A., Chachar, M., Polster, B. M., Brandt, T. A., Fannjiang, Y., Ivanovska, I., Basanez, G., Kinnally, K. W., Zimmerberg, J., Hardwick, J. M., and Kaczmarek, L. K. (2004) Proapoptotic N-truncated BCLxL protein activates endogenous mitochondrial channels in living synaptic terminals, Proc. Natl. Acad. Sci. USA, 101, 13590–13595.
Baggetto, L. G. (1992) Deviant energetic metabolism of glycolytic cancer cells, Biochimie, 74, 959–974.
Newsholme, E., Crabtree, B., and Ardawi, M. (1985) The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells, Biosci. Rep., 400, 393–400.
Kruspig, B., Zhivotovsky, B., and Gogvadze, V. (2014) Mitochondrial substrates in cancer: drivers or passengers? Mitochondrion, 19, Pt. A, 8–19.
Smolkova, K., Plecita-Hlavata, L., Bellance, N., Benard, G., Rossignol, R., and Jezek, P. (2011) Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells, Int. J. Biochem. Cell Biol., 43, 950–968.
Bonnet, S., Archer, S. L., Allalunis-Turner, J., Haromy, A., Beaulieu, C., Thompson, R., Lee, C. T., Lopaschuk, G. D., Puttagunta, L., Bonnet, S., Harry, G., Hashimoto, K., Porter, C. J., Andrade, M. A., Thebaud, B., and Michelakis, E. D. (2007) A mitochondria–K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth, Cancer Cell, 11, 37–51.
Cai, P., Boor, P. J., Khan, M., Kaphalia, B. S., Ansari, G. A., and Konig, R. (2007) Immunoand hepato-toxicity of dichloroacetic acid in MRL+/+ and B6C3F1 mice, J. Immunotoxicol., 4, 107–115.
Moungjaroen, J., Nimmannit, U., Callery, P. S., Wang, L., Azad, N., Lipipun, V., Chanvorachote, P., and Rojanasakul, Y. (2006) Reactive oxygen species mediate caspase activation and apoptosis induced by lipoic acid in human lung epithelial cancer cells through Bcl-2 downregulation, J. Pharmacol. Exp. Ther., 319, 1062–1069.
Simbula, G., Columbano, A., Ledda-Columbano, G. M., Sanna, L., Deidda, M., Diana, A., and Pibiri, M. (2007) Increased ROS generation and p53 activation in alphalipoic acid-induced apoptosis of hepatoma cells, Apoptosis, 12, 113–123.
Feron, O. (2009) Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells, Radiother. Oncol., 92, 329–333.
Dong, L., Low, P., Dyason, J., and Wang, X. (2008) αTocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II, Oncogene, 27, 4324–4335.
Baggetto, L., and Testa-Parussini, R. (1990) Role of acetoin on the regulation of intermediate metabolism of Ehrlich ascites tumor mitochondria: its contribution to membrane cholesterol enrichment modifying passive proton permeability, Arch. Biochem. Biophys., 283, 241–248.
Kruspig, B., Nilchian, A., Bejarano, I., Orrenius, S., Zhivotovsky, B., and Gogvadze, V. (2012) Targeting mitochondria by α-tocopheryl succinate kills neuroblastoma cells irrespective of MycN oncogene expression, Cell. Mol. Life Sci., 69, 2091–2099.
Truksa, J., Dong, L.-F., Rohlena, J., Stursa, J., Vondrusova, M., Goodwin, J., Nguyen, M., Kluckova, K., Rychtarcikova, Z., Lettlova, S., Spacilova, J., Stapelberg, M., Zoratti, M., and Neuzil, J. (2015) Mitochondrially targeted vitamin E succinate modulates expression of mitochondrial DNA transcripts and mitochondrial biogenesis, Antioxid. Redox Signal., 22, 883–900.
Liu, Z., Zhang, Y., Zhang, Q., Zhao, S., Wu, C., Cheng, X., Jiang, C., Jiang, Z., and Liu, H. (2014) 3Bromopyruvate induces apoptosis in breast cancer cells by downregulating Mcl-1 through the PI3K/Akt signaling pathway, Anticancer Drugs, 25, 447–455.
Macchioni, L., Davidescu, M., and Roberti, R. (2014) The energy blockers 3-bromopyruvate and lonidamine: effects on bioenergetics of brain mitochondria, J. Bioenerg. Biomembr., 46, 389–394.
Pereira da Silva, A. P., El-Bacha, T., Kyaw, N., dos Santos, R. S., da Silva, W. S., Almeida, F. C. L., Da Poian, A. T., and Galina, A. (2009) Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate, Biochem. J., 417, 717–726.
Cardaci, S., Rizza, S., Filomeni, G., Bernardini, R., Bertocchi, F., Mattei, M., Paci, M., Rotilio, G., and Ciriolo, M. R. (2012) Glutamine deprivation enhances antitumor activity of 3-bromopyruvate through the stabilization of monocarboxylate transporter-1, Cancer Res., 72, 4526–4536.
Van Delft, M. F., Wei, A. H., Mason, K. D., Vandenberg, C. J., Chen, L., Czabotar, P. E., Willis, S. N., Scott, C. L., Day, C. L., Adams, J. M., Roberts, A. W., and Huang, D. C. S. (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized, Cancer Cell, 10, 389–399.
Fulda, S., Galluzzi, L., and Kroemer, G. (2010) Targeting mitochondria for cancer therapy, Nat. Rev. Drug. Discov., 9, 447–464.
Lessene, G., Czabotar, P. E., and Colman, P. M. (2008) BCL-2 family antagonists for cancer therapy, Nat. Rev. Drug Discov., 7, 989–1000.
Albershardt, T. C., Salerni, B. L., Soderquist, R. S., Bates, D. J., Pletnev, A. A., Kisselev, A. F., and Eastman, A. (2011) Multiple BH3 mimetics antagonize antiapoptotic MCL-1 protein by inducing the endoplasmic reticulum stress response and upregulating BH3-only protein NOXA, J. Biol. Chem., 286, 24882–24895.
Billard, C. (2013) BH3 mimetics: status of the field and new developments, Mol. Cancer Ther., 12, 1691–1700.
Zhang, Z., Song, T., Zhang, T., Gao, J., Wu, G., An, L., and Du, G. (2010) A novel BH3 mimetic S1 potently induces Bax/Bak-dependent apoptosis by targeting both Bcl-2 and Mcl-1, J. Cancer, 128, 1724–1735.
Soderquist, R., Pletnev, A. A., Danilov, A. V., and Eastman, A. (2013) The putative BH3 amimetic S1 sensitizes leukemia to ABT-737 by increasing reactive oxygen species, inducing endoplasmic reticulum stress, and upregulating the BH3-only protein NOXA, Apoptosis, 19, 201–209.
Zhong, J. T., Xu, Y., Yi, H. W., Su, J., Yu, H. M., Xiang, X. Y., Li, X. N., Zhang, Z. C., and Sun, L. K. (2012) The BH3 mimetic S1 induces autophagy through ER stress and disruption of Bcl-2/Beclin 1 interaction in human glioma U251 cells, Cancer Lett., 323, 180–187.
Song, T., Li, X., Chang, X., Liang, X., Zhao, Y., and Wu, G. Y. (2013) 3-Thiomorpholin-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (S1) derivatives as panBcl-2-inhibitors of Bcl-2, Bcl-xL and Mcl-1, Bioorg. Med. Chem., 21, 11–20.
Song, T., Chen, Q., Li, X., Chai, G., and Zhang, Z. C. (2013) Correction to 3-thiomorpholin-8-oxo-8H-acenaphtho[1,2-b]pyrrole-9-carbonitrile (S1)-based molecules as potent, dual inhibitors of B-cell lymphoma 2 (Bcl-2) and myeloid cell leukemia sequence 1 (Mcl-1): structure-based design and structure–activity relationship studies, J. Med. Chem., 56, 9366–9367.
Ponassi, R., Biasotti, B., Tomati, V., Bruno, S., Poggi, A., Malacarne, D., Cimoli, G., Salis, A., Pozzi, S., Miglino, M., Damonte, G., Cozzini, P., Spyraki, F., Campanini, B., Bagnasco, L., Castagnino, N., Tortolina, L., Mumot, A., Frassoni, F., Daga, A., Cilli, M., Piccardi, F., Monfardini, I., Perugini, M., Zoppoli, G., D’Arrigo, C., Pesenti, R., and Parodi, S. (2008) A novel Bim-BH3derived Bcl-XL inhibitor: biochemical characterization, in vitro, in vivo and ex vivo anti-leukemic activity, Cell Cycle, 7, 3211–3224.
Ghiotto, F., Fais, F., Tenca, C., Tomati, V., Morabito, F., Casciaro, S., Mumot, A., Zoppoli, G., Ciccone, E., Parodi, S., and Bruno, S. (2009) Apoptosis of B-cell chronic lymphocytic leukemia cells induced by a novel BH3 peptidomimetic, Cancer Biol. Ther., 8, 263–271.
Cheng, G., Zielonka, J., Dranka, B. P., McAllister, D., Mackinnon, A. C., Jr., Joseph, J., and Kalyanaraman, B. (2012) Mitochondria targeted drugs synergize with 2deoxyglucose to trigger breast cancer cell death, Cancer Res., 72, 2634–2644.
Sahra, I. B., Laurent, K., Giuliano, S., Larbret, F., Ponzio, G., Gounon, P., Le Marchand-Brustel, Y., Giorgetti-Peraldi, S., Cormont, M., Bertolotto, C., Deckert, M., Auberger, P., Tanti, J. F., and Bost, F. (2010) Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells, Cancer Res., 70, 2465–2475.
Zhai, X., Yang, Y., Wan, J., Zhu, R., and Wu, Y. (2013) Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells, Oncol. Rep., 30, 2983–2991.
Meynet, O., Beneteau, M., Jacquin, M. A., Pradelli, L. A., Cornille, A., Carles, M., and Ricci, J.-E. (2012) Glycolysis inhibition targets Mcl-1 to restore sensitivity of lymphoma cells to ABT-737-induced apoptosis, Leukemia, 26, 1145–1147.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2016, Vol. 81, No. 2, pp. 147–165.
Originally published in Biochemistry (Moscow) On-Line Papers in Press as Manuscript BM15-173, December 27, 2015.
Rights and permissions
About this article
Cite this article
Maximchik, P.V., Kulikov, A.V., Zhivotovsky, B.D. et al. Cellular energetics as a target for tumor cell elimination. Biochemistry Moscow 81, 65–79 (2016). https://doi.org/10.1134/S0006297916020012
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916020012