Abstract
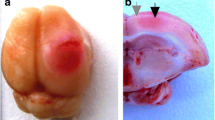
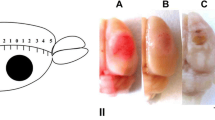
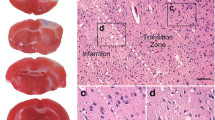
Photodynamic impact on animal cerebral cortex using water-soluble Bengal Rose as a photosensitizer, which does not cross the blood-brain barrier and remains in blood vessels, induces platelet aggregation, vessel occlusion, and brain tissue infarction. This reproduces ischemic stroke. Irreversible cell damage within the infarction core propagates to adjacent tissue and forms a transition zone — the penumbra. Tissue necrosis in the infarction core is too fast (minutes) to be prevented, but much slower penumbral injury (hours) can be limited. We studied the changes in morphology and protein expression profile in penumbra 1 h after local photothrombotic infarction induced by laser irradiation of the cerebral cortex after Bengal Rose administration. Morphological study using standard hematoxylin/eosin staining showed a 3-mm infarct core surrounded by 1.5–2.0 mm penumbra. Morphological changes in the penumbra were lesser and decreased towards its periphery. Antibody microarrays against 224 neuronal and signaling proteins were used for proteomic study. The observed upregulation of penumbra proteins involved in maintaining neurite integrity and guidance (NAV3, MAP1, CRMP2, PMP22); intercellular interactions (N-cadherin); synaptic transmission (glutamate decarboxylase, tryptophan hydroxylase, Munc-18-1, Munc-18-3, and synphilin-1); mitochondria quality control and mitophagy (PINK1 and Parkin); ubiquitin-mediated proteolysis and tissue clearance (UCHL1, PINK1, Parkin, synphilin-1); and signaling proteins (PKBα and ERK5) could be associated with tissue recovery. Downregulation of PKC, PKCβ1/2, and TDP-43 could also reduce tissue injury. These changes in expression of some neuronal proteins were directed mainly to protection and tissue recovery in the penumbra. Some upregulated proteins might serve as markers of protection processes in a penumbra.
Similar content being viewed by others
Abbreviations
- CRMP2:
-
collapsin response mediator protein 2
- DYRK1A:
-
dual-specificity tyrosine-phosphorylated regulated kinase 1A
- ERK5:
-
extracellular regulated kinase 2
- GABA:
-
γ-butyric acid
- MAP1:
-
microtubule-associated protein 1
- NAV3:
-
neuron navigator 3 protein
- PINK1:
-
PTEN-induced mitochondrial protein kinase
- PKBβ1:
-
protein kinase Bα
- PKC:
-
protein kinase C
- PKCβ1:
-
protein kinase C isoform β1
- PMP22:
-
peripheral myelin protein 22
- PTI:
-
photothrombotic infarction
- SIRT1:
-
NAD+-dependent deacetylase sirtuin-1
- TDP-43:
-
transactivation response DNA-binding protein
- UCHL1:
-
ubiquitin C-terminal hydrolase L1
References
Meisel, A., Prass, K., Wolf, T., and Dirnagl, U. (2004) Stroke, in Neuroprotection: Models, Mechanisms and Therapies (Bahr, M., ed.) Wiley-Blackwell, Hoboken, NJ, pp. 9–43.
Iadecola, C., and Anrather, J. (2011) Stroke research at a crossroad: asking the brain for directions, Nat. Neurosci., 14, 1363–1368.
Moskowitz, M. A. (2010) Brain protection: maybe yes, maybe no, Stroke, 41, S85–S86.
Zhiganshina, L. E., and Abakumova, T. R. (2013) Cerebrolysin in a treatment of acute ischemic stroke, Vestnik RAMN, 1, 21–29.
Watson, B. D., Dietrich, W. D., Busto, R., Wachtel, M. S., and Ginsberg, M. D. (1985) Induction of reproducible brain infarction by photochemically initiated thrombosis, Ann. Neurol., 17, 497–504.
Dietrich, W. D., Watson, B. D., Busto, R., Ginsberg, M. D., and Bethea, J. R. (1987) Photochemically induced cerebral infarction. I. Early microvascular alterations, Acta Neuropathol., 72, 315–325.
Pevsner, P. H., Eichenbaum, J. W., Miller, D. C., Pivawer, G., Eichenbaum, K. D., Stern, A., Zakian, K. L., and Koutcher, J. A. (2001) A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation, J. Pharmacol. Toxicol. Methods, 45, 227–233.
Shanina, E. V., Redecker, C., Reinecke, S., Schallert, T., and Witte, O. W. (2005) Long-term effects of sequential cortical infarcts on scar size, brain volume and cognitive function, Behav. Brain Res., 158, 69–77.
Schmidt, A., Hoppen, M., Strecker, J. K., Diederich, K., Schabitz, W. R., Schilling, M., and Minnerup, J. (2012) Photochemically induced ischemic stroke in rats, Exp. Transl. Stroke Med., 4, 13.
Romanova, G. A., Barskov, I. V., Ostrovskaya, R. U., Gudasheva, T. A., and Viktorov, I. V. (1998) Behavioral and morphological changes induced by bilateral photoinduced thrombosis of cerebral vessels in the rat frontal cortex, Pathol. Physiol. Exp. Ther., No. 2, 8–10.
Romanova, G. A., Shakova, F. M., Barskov, I. V., Stelmashuk, E. V., Genrihs, E. E., Cheremnyh, A. M., Kalinina, T. I., and Yurin, V. L. (2014) Neuroprotective and anti-amnestic effect of erythropoietin derivatives at experimental ischemic damage to brain cortex, Bull. Exp. Biol. Med., 158, 299–302.
Uzdensky, A. B. (2010) Cellular and Molecular Mechanisms of Photodynamic Therapy [in Russian], Nauka, St. Petersburg.
Brundel, M., de Bresser, J., van Dillen, J. J., Kappelle, L. J., and Biessels, G. J. (2012) Cerebral microinfarcts: a systematic review of neuropathological studies, J. Cereb. Blood Flow Metab., 32, 425–436.
Pantoni, L. (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges, Lancet Neurol., 9, 689–701.
Del Zoppo, G. J., and Mabuchi, T. (2003) Cerebral microvessel responses to focal ischemia, J. Cereb. Blood Flow Metab., 23, 879–894.
Spisak, S., Tulassay, Z., Molnar, B., and Guttman, A. (2007) Protein microchips in biomedicine and biomarker discovery, Electrophoresis, 28, 4261–4273.
Wingren, C., and Borrebaeck, C. A. (2009) Antibody-based microarrays, Methods Mol. Biol., 509, 57–84.
Dayon, L., Turck, N., Garci-Berrocoso, T., Walter, N., Burkhard, P. R., Vilalta, A., Sahuquillo, J., Montaner, J., and Sanchez, J. C. (2011) Brain extracellular fluid protein changes in acute stroke patients, J. Proteome Res., 10, 1043–1051.
Demyanenko, S. V., Uzdensky, A. B., Sharifulina, S. A., Lapteva, T. O., and Polyakova, L. P. (2014) PDT-induced epigenetic changes in the mouse cerebral cortex: a protein microarray study, Biochim. Biophys. Acta, 1840, 262–270.
Zilles, K. (1985) The Cortex of the Rat: A Stereotaxis Atlas, Springer-Verlag, Berlin.
Villa, R. F., Gorini, A., Ferrari, F., and Hoyer, S. (2013) Energy metabolism of cerebral mitochondria during aging, ischemia and post-ischemic recovery assessed by functional proteomics of enzymes, Neurochem. Int., 63, 765–781.
Datta, A., Park, J. E., Li, X., Zhang, H., Ho, Z. S., Heese, K., Lim, S. K., Tam, J. P., and Sze, S. K. (2010) Phenotyping of an in vitro model of ischemic penumbra by iTRAQ-based shotgun quantitative proteomics, J. Proteom. Res., 9, 472–484.
Bu, X., Zhang, N., Yang, X., Liu, Y., Du, J., Liang, J., Xu, Q., and Li, J. (2011) Proteomic analysis of PKCßII-interacting proteins involved in HPC-induced neuroprotection against cerebral ischemia of mice, J. Neurochem., 117, 346–356.
Hara, H., Onodera, H., Yoshidomi, M., Matsuda, Y., and Kogure, K. (1990) Staurosporine, a novel protein kinase C inhibitor, prevents postischemic neuronal damage in the gerbil and rat, J. Cereb. Blood Flow Metab., 10, 646–653.
Felipo, V., Minana, M. D., and Grisolia, S. (1993) Inhibitors of protein kinase C prevent the toxicity of glutamate in primary neuronal cultures, Brain Res., 604, 192–196.
Bright, R., and Mochly-Rosen, D. (2005) The role of protein kinase C in cerebral ischemic and reperfusion injury, Stroke, 36, 2781–2790.
Chou, W. H., and Messing, R. O. (2005) Protein kinase C isozymes in stroke, Trends Cardiovasc. Med., 15, 47–51.
Lee, B. K., Yoon, J. S., Lee, M. G., and Jung, Y. S. (2014) Protein kinase C-β mediates neuronal activation of Na+/H+ exchanger-1 during glutamate excitotoxicity, Cell Signal., 26, 697–704.
Wang, J., Bright, R., Mochly-Rosen, D., and Giffard, R. G. (2004) Cell-specific role for e-and βI-protein kinase C isozymes in protecting cortical neurons and astrocytes from ischemia-like injury, Neuropharmacology, 47, 136–145.
Zhao, H., Sapolsky, R. M., and Steinberg, G. K. (2006) Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke, Mol. Neurobiol., 34, 249–270.
Wang, R. M., Zhang, Q. G., Li, C. H., and Zhang, G. Y. (2005) Activation of extracellular signal-regulated kinase 5 may play a neuroprotective role in hippocampal CA3/DG region after cerebral ischemia, J. Neurosci. Res., 80, 391–399.
Laguna, A., Aranda, S., Barallobre, M. J., Barhoum, R., Fernandez, E., Fotaki, V., Delabar, J. M., de la Luna, S., Villa, P., and Arbones, M. L. (2008) The protein kinase DYRK1A regulates caspase-9-mediated apoptosis during retina development, Dev. Cell, 15, 841–853.
Choi, H. K., and Chung, K. C. (2011) DYRK1A positively stimulates ASK1-JNK signaling pathway during apoptotic cell death, Exp. Neurobiol., 20, 35–44.
Guo, X., Williams, J. G., Schug, T. T., and Li, X. (2010) DYRK1A and DYRK3 promote cell survival through phosphorylation and activation of SIRT1, J. Biol. Chem., 285, 3223–3232.
Trancikova, A., Tsika, E., and Moore, D. J. (2012) Mitochondrial dysfunction in genetic animal models of Parkinson’s disease, Antioxid. Redox Signal., 16, 896–919.
De Vries, R. L., and Przedborski, S. (2013) Mitophagy and Parkinson’s disease: be eaten to stay healthy, Mol. Cell Neurosci., 55, 37–43.
Caldeira, M. V., Salazar, I. L., Curcio, M., Canzoniero, L. M., and Duarte, C. B. (2014) Role of the ubiquitin-proteasome system in brain ischemia: friend or foe? Prog. Neurobiol., 112, 50–69.
Yamauchi, T., Sakurai, M., Abe, K., Matsumiya, G., and Sawa, Y. (2008) Ubiquitin-mediated stress response in the spinal cord after transient ischemia, Stroke, 39, 1883–1889.
Kruger, R. (2004) The role of synphilin-1 in synaptic function and protein degradation, Cell Tissue Res., 318, 195–199.
Maes, T., Barcelo, A., and Buesa, C. (2002) Neuron navigator: a human gene family with homology to unc-53, a cell guidance gene from Caenorhabditis elegans, Genomics, 80, 21–30.
Halpain, S., and Dehmelt, L. (2006) The MAP1 family of microtubule-associated proteins, Genome Biol., 7, 2–4.
Chen, A., Liao, W. P., Lu, Q., Wong, W. S., and Wong, P. T. (2007) Up-regulation of dihydropyrimidinase-related protein 2, spectrin alpha II chain, heat shock cognate protein 70 pseudogene 1 and tropomodulin 2 after focal cerebral ischemia in rats — a proteomics approach, Neurochem. Int., 50, 1078–1086.
Hou, S. T., Jiang, S. X., Aylsworth, A., Ferguson, G., Slinn, J., Hu, H., Leung, T., Kappler, J., and Kaibuchi, K. (2009) CaMKII phosphorylates collapsin response mediator protein 2 and modulates axonal damage during glutamate excitotoxicity, J. Neurochem., 111, 870–881.
Quarles, R. H. (2002) Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration, Cell. Mol. Life Sci., 59, 1851–1871.
Gallwitz, D., and Jahn, R. (2003) The riddle of the Sec1/Munc-18 proteins — new twists added to their interactions with SNAREs, Trends Biochem. Sci., 28, 113–116.
Lee, E. B., Lee, V. M., and Trojanowski, J. Q. (2011) Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration, Nat. Rev. Neurosci., 13, 38–50.
Kanazawa, M., Kakita, A., Igarashi, H., Takahashi, T., Kawamura, K., Takahashi, H., Nakada, T., Nishizawa, M., and Shimohata, T. (2011) Biochemical and histopathological alterations in TAR DNA-binding protein-43 after acute ischemic stroke in rats, J. Neurochem., 116, 957–965.
Zechariah, A. E., Ali, A., Hagemann, N., Jin, F., Doeppner, T. R., Helfrich, I., Mies, G., and Hermann, D. M. (2013) Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells, Arterioscler. Thromb. Vasc. Biol., 33, 1561–1567.
Back, T., Ginsberg, M. D., Dietrich, W. D., and Watson, B. D. (1996) Induction of spreading depression in the ischemic hemisphere following experimental middle cerebral artery occlusion: effect on infarct morphology, J. Cereb. Blood Flow Metab., 16, 202–213.
Puyal, J., Ginet, V., and Clarke, P. G. (2013) Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection, Prog. Neurobiol., 105, 24–48.
Sims, N. R., and Anderson, M. F. (2002) Mitochondrial contributions to tissue damage in stroke, Neurochem. Int., 40, 511–526.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2015, Vol. 80, No. 6, pp. 937–948.
Rights and permissions
About this article
Cite this article
Demyanenko, S.V., Panchenko, S.N. & Uzdensky, A.B. Expression of neuronal and signaling proteins in penumbra around a photothrombotic infarction core in rat cerebral cortex. Biochemistry Moscow 80, 790–799 (2015). https://doi.org/10.1134/S0006297915060152
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915060152