Summary
MAP1-family proteins are classical microtubule-associated proteins (MAPs) that bind along the microtubule lattice. The founding members, MAP1A and MAP1B, are predominantly expressed in neurons, where they are thought to be important in the formation and development of axons and dendrites. Mammalian genomes usually contain three family members, MAP1A, MAP1B and a shorter, more recently identified gene called MAP1S. By contrast, only one family member, Futsch, is found in Drosophila. After their initial expression, the MAP1A and MAP1B polypeptides are cleaved into light and heavy chains, which are then assembled into mature complexes together with the separately encoded light chain 3 subunit (LC3). Both MAP1A and MAP1B are well known for their microtubule-stabilizing activity, but MAP1 proteins can also interact with other cellular components, including filamentous actin and signaling proteins. Furthermore, the activity of MAP1A and MAP1B is controlled by upstream signaling mechanisms, including the MAP kinase and glycogen synthase kinase-3 β pathways.
Similar content being viewed by others
Gene organization and evolutionary history
Various classes of microtubule-associated proteins (MAPs) are expressed in eukaryotic cells. Whereas some MAPs bind specifically to the microtubule plus ends or the minus ends (centrosomes), many MAPs bind along the microtubule lattice. The latter category includes both enzymatically active MAPs, such as microtubule motors or the microtubule-severing protein katanin, and structural MAPs such as the MAP2/tau or stable tubule only (STOP) protein families. This article focuses on the classical, microtubule lattice-binding structural MAPs in the MAP1 family, which are best known for their microtubule-stabilizing activity. Most knowledge on MAP1-family proteins has been derived from studies in rodents and, unless noted otherwise, insights from rodents are expected to apply for mammalian family members in general. The MAP2/tau family of classical MAPs is encoded by distinct, apparently unrelated genes and has been reviewed in an earlier issue of Genome Biology [1].
Most vertebrate genomes (including human, mouse and rat) contain three family members, MAP1A, MAP1B and MAP1S, which are encoded by separate genes (see Table 1 for chromosomal locations of the human genes) [2–4]. The shortest MAP1 protein, MAP1S, is also known as VCY2IP1 or C19ORF5. A search for proteins with sequence similarity to MAP1A or MAP1B proteins (summarized in Figure 1a) shows that there are three apparent MAP1 family members in bony fish (L.D. and S.H., unpublished observations), but the functional significance of these isoforms is unclear. Fish MAP1-family proteins have been reported to be only about 25% of the size of their mammalian counterparts [5]. No obvious ortholog of any MAP1-family protein is present in Caenorhabditis elegans or more primitive organisms, but a single protein related to MAP1A and MAP1B, called Futsch, can be found in Drosophila melanogaster [6]. Futsch differs from vertebrate MAP1A and MAP1B isoforms in that it contains a repeated central domain with homology to vertebrate neurofilaments [6]. As classic neurofilaments are absent from the Drosophila genome, it may be that Futsch is an ancestral precursor of neurofilament proteins.
Phylogenetic analysis of (a) MAP1 and (b) LC3 family proteins. LC3-related proteins do not share significant sequence homology with any of the MAP1 family members; phylogenetic relationships of the two families were therefore analyzed separately using Phylip [60]. Drosophila Futsch and the family members found by sequence analysis from the pufferfish Tetraodon nigroviridis cannot be definitively assigned as orthologs to any one mammalian protein.
Vertebrate MAP1-family genes span multiple exons. Alternative splicing has been reported only in mammalian MAP1B genes [3]; its functional relevance is unclear. Sequence similarity between distinct MAP1-family proteins in an individual organism is most prominent in the extreme amino and carboxyl termini (approximately 85% similarity at the amino-acid level). Sequences with significant similarities are also found in Drosophila Futsch (approximately 60% similarity to rat MAP1A or MAP1B), but it is not clear from this information whether the Drosophila protein is an ortholog of either MAP1A or MAP1B (Figure 1a).
An accessory protein chain that can be found in MAP1A and MAP1B protein complexes is derived from the LC3 gene encoding MAP1 light chain 3 [7]. LC3 and related proteins do not show significant sequence similarity to MAP1A and MAP1B and are not usually considered to be part of the MAP1 protein family. At least seven distinct LC3-related genes are found in humans (Table 1, Figure 1b), and various orthologs of these genes are found in both highly developed and simpler eukaryotes. LC3-related genes are related to the yeast ubiquitin-like gene AUT7 (ATG8) [8] and are thought to play a role in autophagy. One LC3-related gene has been predicted in archaea (hypothetical protein ST0261 in Sulfolobus tokodaii). No orthologs of the MAP1 and LC3 families are found in prokaryotes.
Characteristic structural features
The MAP1A, MAP1B and MAP1S polypeptides are each translated as larger proteins that are then processed by proteolytic cleavage near the carboxyl terminus, leading to the generation of heavy chains (MAP1A-HC of 350 kDa, MAP1B-HC of 300 kDa and MAP1S-HC of 100 kDa) and light chains (LC2 of 28 kDa from MAP1A, LC1 of 32 kDa from MAP1B and MAP1S-LC of 26 kDa) [4, 9, 10]. The light chains generated by MAP1A (LC2) and MAP1B (LC1) can interact with both MAP1A and MAP1B heavy chains [11]. For MAP1B, light-chain binding has been mapped to a 120 kDa fragment within the amino terminus of the heavy chain [10]. LC3 can also interact with the MAP1A and MAP1B heavy chains [7]. The exact stoichiometric composition of MAP1A and MAP1B heavy and light chains has not been determined, but in the case of MAP1B, a ratio of MAP1B-HC:LC1:LC3 of 1:2:0.2 has been estimated [12].
All four light chains can bind microtubules by themselves [4, 7, 13], and the MAP1A and MAP1B heavy chains both contain additional sequences that bind microtubules (Figure 2) [14–17]. Amino acids within these microtubule-binding domains are diverse, including both positively and negatively charged residues. Interestingly, the MAP1B heavy chain was found to suppress the microtubule-binding activity of its light chain [18]. For MAP1A, the contributions of the heavy and light chains for microtubule binding are less clear. In kangaroo PtK2 cells, exogenously expressed MAP1A light chain (LC2) was sufficient by itself to bind and stabilize microtubules [13]. In contrast, in green monkey COS7 cells, both MAP1A light and heavy chains were required [19].
Domain organization and posttranslational processing of mammalian MAP1-family proteins. (a) MAP1A, MAP1B and MAP1S contain microtubule- and F-actin-binding sequences in their carboxyl termini, and additional microtubule-binding sites have been mapped to the amino termini of MAP1A and MAP1B. The first microtubule-binding motif of MAP1A and MAP1B include several basic repeats of the amino-acid sequence KKE. In the case of MAP1A, it has been suggested that sequences in the regions flanking these repeats can bind microtubules by themselves [17]. However, the exact location of all sequences involved in this activity has not been mapped to date. All mammalian family members are cleaved near their carboxyl terminus into heavy and light chains. (b) A schematic representation of the posttranslational processing of MAP1A and MAP1B. Black arrows denote preferential interactions; gray arrows denote possible interactions. Once formed, the light chains of MAP1A or MAP1B can interact with the heavy chains of either MAP1A or MAP1B, but a preference for the MAP1A-derived light chain LC2 to bind MAP1A heavy chain has been noted [11]. A separate gene encodes an additional light chain, LC3, which is also found in mature MAP1A or MAP1B complexes.
In addition to microtubule-binding activity, the MAP1A, MAP1B and MAP1S light chains can also bind filamentous actin (F-actin) [4, 13, 18]. The microtubule- and F-actin-binding sites on the MAP1A and MAP1B light chains map to different sequence regions. Microtubule binding is confined to the amino terminus of the light chains, and a direct F-actin interaction has been localized to the carboxyl terminus. Furthermore, an exogenous carboxy-terminal fragment of MAP1A and MAP1B light chains colocalized with F-actin in stress fibers of non-neuronal cells [13, 18]. It is yet not known if a single MAP1 unit can bind both cytoskeletons at the same time and thus crosslink the two cytoskeletons.
Structural details about MAP1-family proteins are largely unknown. Both microtubule and F-actin binding have been mapped to regions of about 120 amino acids in the MAP1B light chain, but no further structural details or critical amino-acid residues related to these interactions have been identified. The only structural data available are derived from electron microscopy of preparations treated by the rotary shadowing technique. These studies suggest that MAP1A is a flexible, elongated protein [20], whereas MAP1B appears to be a rod-shaped, elongated molecule with a terminal, round globular domain [21]. No information about secondary structures in either molecule is available. Moreover, predictions suggest that mammalian MAP1A and MAP1B and Drosophila Futsch are natively unfolded (L.D. and S.H., unpublished observations; predictions were calculated using FoldIndex [22]). Although over 50% of their entire protein sequences are predicted to be unstructured, some folded regions might exist in the extreme amino termini of the heavy chains.
Localization and function
MAP1 family members and their splice variants have specific regional and temporal expression patterns in the nervous system. MAP1B is highly expressed during early neuronal development and gradually diminishes during maturation [23]. In developing cultured neurons, MAP1B protein is localized to axons, as well as their precursors (so-called 'minor neurites') [24]. Furthermore, MAP1B is especially enriched in growing axons [23, 24]. MAP1A is predominantly expressed in adult neurons, where it localizes preferentially to dendrites [11]. MAP1S is expressed in various tissues including mouse brain [4].
Functions of MAP1A and MAP1B in the nervous system
MAP1A and MAP1B were originally discovered because of, and were characterized by, their ability to bind and stabilize micro-tubules. Ultrastructural analysis revealed the presence of these MAPs along the sides of microtubules [20, 21]. In vitro studies suggested that the microtubule-stabilizing activity of MAP1B is weaker than that of the distinct neuronal microtubule stabilizer MAP2 [25]. This could be a consequence of factors such as differential phosphorylation or the recently documented inhibition of microtubule-stabilizing activity of MAP1B light chain by its heavy chain [18]. Overexpression of MAP1B in heterologous cell systems induces the formation of micro-tubule bundles with a 'wavy' appearance [13]. In contrast, microtubule bundles induced by MAPs of the MAP2/tau family are straight and rigid [26, 27]. Evidence for direct crosslinking of microtubules by MAP1A and MAP1B is lacking, leaving open a potential role for adapter proteins.
Complete removal of the MAP1B gene results in the absence of the corpus callosum [28], a brain region mostly composed of axons that cross the midline, suggesting that certain axonal growth mechanisms are disturbed. More recently, enhanced neurite branching and impaired axonal turning behavior were reported in regenerating adult mouse dorsal root ganglion neurons lacking MAP1B [29]. Earlier reports of various knockout lines in which only portions of the MAP1B gene were removed described either more severe [30, 31] or less severe [32] phenotypes, presumably owing to different genetic backgrounds or different alternatively spliced MAP1B isoforms.
Interestingly, functional redundancy of MAP1B with both MAPs of the MAP2/tau family has been reported [33–35]. Simultaneous inhibition of MAP1B and either MAP2 or tau resulted in more severe phenotypes than single knockouts. Taken together, these experiments suggest a role for MAP1B, tau and MAP2 in both neuronal migration and process outgrowth. Knockout studies of the MAP1A and MAP1S genes have not been reported to date. Other classes of MAPs have functions that at least partially overlap with those of the MAP1 and MAP2/tau families: proteins such as STOP, adenomatous polyposis coli (APC), doublecortin, or spectraplakins might provide additional redundancy in MAP function.
Role of MAP1A and MAP1B as adaptor proteins
The MAP1-family proteins have been shown to interact with numerous proteins and specific functions have been proposed for some of these interactions. For example, MAP1A is found in postsynaptic densities (PSDs), where it interacts with PSD-95. This interaction might be functionally important: mutations that reduce the MAP1A-PSD95 interaction confer sensitivity to hearing loss induced by a mutation in the tub gene, a condition that is proposed to involve defects in synaptic function [36]. More recently, the interaction between MAP1B and the disease-related protein gigaxonin has been suggested to be critically involved in the progression of giant axonal neuropathy, a human neurodegenerative disease [37]. Table 2 provides an overview of identified interaction partners and briefly describes the proposed function of each interaction.
Mechanism and regulation
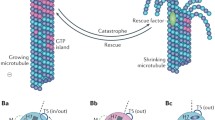
Microtubules exhibit dynamic instability, an intrinsic behavior characterized by alternating phases of growth, shortening and pausing. MAP1A and MAP1B proteins bind along the length of microtubules and are thought to stabilize micro-tubules by altering this dynamic behavior. One study suggests that MAP1B mediates microtubule stabilization specifically by reducing depolymerization rates [25].
Phosphorylation of MAP1B by glycogen synthase kinase-3 β (GSK3β) has been extensively studied both in vitro and in vivo. Phosphorylation by this kinase has been mapped to two residues, Ser1260 and Thr1265, which are specifically phosphorylated in growing axons [38]. Furthermore, in contrast to the microtubule-stabilizing effect of unphosphorylated MAP1B, GSK3β-phosphorylated MAP1B sensitizes microtubules to depolymerizing agents [39]. Taken together, such experiments lead to the idea that MAP1B's phosphorylation state might regulate microtubule stability in growing axons, and thereby influence axonal growth.
Recent evidence also links the Jun N-terminal kinase (JNK) pathway to phosphorylation of MAP1B [40]. Less is known about MAP1A, but a recent study suggests that activity-dependent dendritic remodeling through the mitogen-activated protein (MAP) kinase pathway is dependent on MAP1A [41]. Very little is known about the mechanism and regulation of MAP1S.
Frontiers
Two decades after their original discovery, many functions of MAP1A and MAP1B have been uncovered in vitro and in vivo. Knockout animals and functional assays suggest specific roles of MAP1 family members in both the development and the degeneration of the nervous system. Structural details of MAP1-family proteins are largely unknown, however, and apparent functional redundancies and crosstalk with other MAPs and cytoskeletal regulators make it difficult to pinpoint the exact function(s) of individual MAPs in vivo. Furthermore, the variety of upstream regulatory pathways and downstream effectors provide a major challenge to fully understanding MAP1A and MAP1B function. Fortunately, certain key pathways controlling MAP1A and MAP1B activity have been identified, although little is yet known about MAP1S. A broader and more precise analysis of phosphorylation and other posttranslational modifications still needs to be carried out, however, in order to fully understand MAP1A and MAP1B function in signaling networks controlling neuromorphogenesis.
References
Dehmelt L, Halpain S: The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005, 6: 204-10.1186/gb-2004-6-1-204.
Fink JK, Jones SM, Esposito C, Wilkowski J: Human microtubule-associated protein 1a (MAP1A) gene: genomic organization, cDNA sequence, and development. Genomics. 1996, 35: 577-585. 10.1006/geno.1996.0400.
Kutschera W, Zauner W, Wiche G, Propst F: The mouse and rat MAP1B genes: genomic organization and alternative transcription. Genomics. 1998, 49: 430-436. 10.1006/geno.1998.5294.
Orban-Nemeth Z, Simader H, Badurek S, Trancikova A, Propst F: Microtubule-associated protein 1S, a short and ubiquitously expressed member of the microtubule-associated protein 1 family. J Biol Chem. 2005, 280: 2257-2265. 10.1074/jbc.M408984200.
Tomasiewicz HG, Wood JG: Characterization of microtubule-associated proteins in teleosts. Cell Motil Cytoskeleton. 1999, 44: 155-167. 10.1002/(SICI)1097-0169(199911)44:3<155::AID-CM1>3.0.CO;2-9.
Hummel T, Krukkert K, Roos J, Davis G, Klambt C: Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000, 26: 357-370. 10.1016/S0896-6273(00)81169-1.
Mann SS, Hammarback JA: Molecular characterization of light chain 3. A microtubule binding subunit of MAP1A and MAP1B. J Biol Chem. 1994, 269: 11492-11497.
Schwartz DC, Hochstrasser M: A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem Sci. 2003, 28: 321-328. 10.1016/S0968-0004(03)00113-0.
Langkopf A, Hammarback JA, Muller R, Vallee RB, Garner CC: Microtubule-associated proteins 1A and LC2. Two proteins encoded in one messenger RNA. J Biol Chem. 1992, 267: 16561-16566.
Hammarback JA, Obar RA, Hughes SM, Vallee RB: MAP1B is encoded as a polyprotein that is processed to form a complex N-terminal microtubule-binding domain. Neuron. 1991, 7: 129-139. 10.1016/0896-6273(91)90081-A.
Schoenfeld TA, McKerracher L, Obar R, Vallee RB: MAP 1A and MAP 1B are structurally related microtubule associated proteins with distinct developmental patterns in the CNS. J Neurosci. 1989, 9: 1712-1730.
Pedrotti B, Islam K: Purification of microtubule associated protein MAP1B from bovine brain: MAP1B binds to micro-tubules but not to microfilaments. Cell Motil Cytoskeleton. 1995, 30: 301-309. 10.1002/cm.970300407.
Noiges R, Eichinger R, Kutschera W, Fischer I, Nemeth Z, Wiche G, Propst F: Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties. J Neurosci. 2002, 22: 2106-2114.
Cravchik A, Reddy D, Matus A: Identification of a novel micro-tubule-binding domain in microtubule-associated protein 1A (MAP1A). J Cell Sci. 1994, 107: 661-672.
Noble M, Lewis SA, Cowan NJ: The microtubule binding domain of microtubule-associated protein MAP1B contains a repeated sequence motif unrelated to that of MAP2 and tau. J Cell Biol. 1989, 109: 3367-3376. 10.1083/jcb.109.6.3367.
Zauner W, Kratz J, Staunton J, Feick P, Wiche G: Identification of two distinct microtubule binding domains on recombinant rat MAP 1B. Eur J Cell Biol. 1992, 57: 66-74.
Vaillant AR, Muller R, Langkopf A, Brown DL: Characterization of the microtubule-binding domain of microtubule-associated protein 1A and its effects on microtubule dynamics. J Biol Chem. 1998, 273: 13973-81. 10.1074/jbc.273.22.13973.
Togel M, Wiche G, Propst F: Novel features of the light chain of microtubule-associated protein MAP1B: microtubule stabilization, self interaction, actin filament binding, and regulation by the heavy chain. J Cell Biol. 1998, 143: 695-707. 10.1083/jcb.143.3.695.
Chien CL, Lu KS, Lin YS, Hsieh CJ, Hirokawa N: The functional cooperation of MAP1A heavy chain and light chain 2 in the binding of microtubules. Exp Cell Res. 2005, 308: 446-458. 10.1016/j.yexcr.2005.05.007.
Shiomura Y, Hirokawa N: The molecular structure of micro-tubule-associated protein 1A (MAP1A) in vivo and in vitro. An immunoelectron microscopy and quick-freeze, deep-etch study. J Neurosci. 1987, 7: 1461-1469.
Sato-Yoshitake R, Shiomura Y, Miyasaka H, Hirokawa N: Micro-tubule-associated protein 1B: molecular structure, localization, and phosphorylation-dependent expression in developing neurons. Neuron. 1989, 3: 229-238. 10.1016/0896-6273(89)90036-6.
Prilusky J, Felder CE, Zeev-Ben-Mordehai T, Rydberg EH, Man O, Beckmann JS, Silman I, Sussman JL: FoldIndex: a simple tool to predict whether a given protein sequence is intrinsically unfolded. Bioinformatics. 2005, 21: 3435-3438. 10.1093/bioinformatics/bti537.
Tucker RP, Matus AI: Developmental regulation of two micro-tubule-associated proteins (MAP2 and MAP5) in the embryonic avian retina. Development. 1987, 101: 535-546.
Denny JB: MAP5 in cultured hippocampal neurons: expression diminishes with time and growth cones are not immunostained. J Neurocytol. 1991, 20: 627-636. 10.1007/BF01187065.
Vandecandelaere A, Pedrotti B, Utton MA, Calvert RA, Bayley PM: Differences in the regulation of microtubule dynamics by microtubule-associated proteins MAP1B and MAP2. Cell Motil Cytoskeleton. 1996, 35: 134-146. 10.1002/(SICI)1097-0169(1996)35:2<134::AID-CM6>3.0.CO;2-A.
Felgner H, Frank R, Biernat J, Mandelkow EM, Mandelkow E, Ludin B, Matus A, Schliwa M: Domains of neuronal microtubule-associated proteins and flexural rigidity of microtubules. J Cell Biol. 1997, 138: 1067-1075. 10.1083/jcb.138.5.1067.
Lewis SA, Ivanov IE, Lee GH, Cowan NJ: Organization of micro-tubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP2 and tau. Nature. 1989, 342: 498-505. 10.1038/342498a0.
Meixner A, Haverkamp S, Wassle H, Fuhrer S, Thalhammer J, Kropf N, Bittner RE, Lassmann H, Wiche G, Propst F: MAP1B is required for axon guidance and is involved in the development of the central and peripheral nervous system. J Cell Biol. 2000, 151: 1169-1178. 10.1083/jcb.151.6.1169.
Bouquet C, Soares S, von Boxberg Y, Ravaille-Veron M, Propst F, Nothias F: Microtubule-associated protein 1B controls directionality of growth cone migration and axonal branching in regeneration of adult dorsal root ganglia neurons. J Neurosci. 2004, 24: 7204-7213. 10.1523/JNEUROSCI.2254-04.2004.
Edelmann W, Zervas M, Costello P, Roback L, Fischer I, Hammarback JA, Cowan N, Davies P, Wainer B, Kucherlapati R: Neuronal abnormalities in microtubule-associated protein 1B mutant mice. Proc Natl Acad Sci USA. 1996, 93: 1270-1275. 10.1073/pnas.93.3.1270.
Gonzalez-Billault C, Demandt E, Wandosell F, Torres M, Bonaldo P, Stoykova A, Chowdhury K, Gruss P, Avila J, Sanchez MP: Perinatal lethality of microtubule-associated protein 1B-deficient mice expressing alternative isoforms of the protein at low levels. Mol Cell Neurosci. 2000, 16: 408-421. 10.1006/mcne.2000.0880.
Takei Y, Kondo S, Harada A, Inomata S, Noda T, Hirokawa N: Delayed development of nervous system in mice homozygous for disrupted microtubule-associated protein 1B (MAP1B) gene. J Cell Biol. 1997, 137: 1615-1626. 10.1083/jcb.137.7.1615.
DiTella MC, Feiguin F, Carri N, Kosik KS, Caceres A: MAP-1B/TAU functional redundancy during laminin-enhanced axonal growth. J Cell Sci. 1996, 109: 467-477.
Takei Y, Teng J, Harada A, Hirokawa N: Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol. 2000, 150: 989-1000. 10.1083/jcb.150.5.989.
Teng J, Takei Y, Harada A, Nakata T, Chen J, Hirokawa N: Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization. J Cell Biol. 2001, 155: 65-76. 10.1083/jcb.200106025.
Ikeda A, Zheng QY, Zuberi AR, Johnson KR, Naggert JK, Nishina PM: Microtubule-associated protein 1A is a modifier of tubby hearing (moth1). Nat Genet. 2002, 30: 401-405. 10.1038/ng838.
Allen E, Ding J, Wang W, Pramanik S, Chou J, Yau V, Yang Y: Gigaxonin-controlled degradation of MAP1B light chain is critical to neuronal survival. Nature. 2005, 438: 224-228. 10.1038/nature04256.
Trivedi N, Marsh P, Goold RG, Wood-Kaczmar A, Gordon-Weeks PR: Glycogen synthase kinase-3beta phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. J Cell Sci. 2005, 118: 993-1005. 10.1242/jcs.01697.
Goold RG, Owen R, Gordon-Weeks PR: Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J Cell Sci. 1999, 112: 3373-3384.
Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M: JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003, 4: 521-533. 10.1016/S1534-5807(03)00094-7.
Szebenyi G, Bollati F, Bisbal M, Sheridan S, Faas L, Wray R, Haferkamp S, Nguyen S, Caceres A, Brady ST: Activity-driven dendritic remodeling requires microtubule-associated protein 1A. Curr Biol. 2005, 15: 1820-1826. 10.1016/j.cub.2005.08.069.
Gupta M, Yarwood SJ: MAP1A light chain 2 interacts with exchange protein activated by cyclic AMP 1 (EPAC1) to enhance Rap1 GTPase activity and cell adhesion. J Biol Chem. 2005, 280: 8109-8116. 10.1074/jbc.M413697200.
Morris JA, Kandpal G, Ma L, Austin CP: DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003, 12: 1591-1608. 10.1093/hmg/ddg162.
Brenman JE, Topinka JR, Cooper EC, McGee AW, Rosen J, Milroy T, Ralston HJ, Bredt DS: Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A. J Neurosci. 1998, 18: 8805-8813.
Wolff S, Xiao Z, Wittau M, Sussner N, Stoter M, Knippschild U: Interaction of casein kinase 1 delta (CK1 delta) with the light chain LC2 of microtubule associated protein 1A (MAP1A). Biochim Biophys Acta. 2005, 1745: 196-206.
Park SM, Liu G, Kubal A, Fury M, Cao L, Marx SO: Direct interaction between BKCa potassium channel and microtubule-associated protein 1A. FEBS Lett. 2004, 570: 143-148. 10.1016/j.febslet.2004.06.037.
Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N: Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci. 1992, 103: 953-964.
Pedrotti B, Islam K: Dephosphorylated but not phosphorylated microtubule associated protein MAP1B binds to microfila-ments. FEBS Lett. 1996, 388: 131-133. 10.1016/0014-5793(96)00520-0.
Opal P, Garcia JJ, Propst F, Matilla A, Orr HT, Zoghbi HY: Map-modulin/leucine-rich acidic nuclear protein binds the light chain of microtubule-associated protein 1B and modulates neuritogenesis. J Biol Chem. 2003, 278: 34691-34699. 10.1074/jbc.M302785200.
Ding J, Liu JJ, Kowal AS, Nardine T, Bhattacharya P, Lee A, Yang Y: Microtubule-associated protein 1B: a neuronal binding partner for gigaxonin. J Cell Biol. 2002, 158: 427-433. 10.1083/jcb.200202055.
Franzen R, Tanner SL, Dashiell SM, Rottkamp CA, Hammer JA, Quarles RH: Microtubule-associated protein 1B: a neuronal binding partner for myelin-associated glycoprotein. J Cell Biol. 2001, 155: 893-898. 10.1083/jcb.200108137.
Hanley JG, Koulen P, Bedford F, Gordon-Weeks PR, Moss SJ: The protein MAP-1B links GABA(C) receptors to the cytoskeleton at retinal synapses. Nature. 1999, 397: 66-69. 10.1038/16258.
Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K: Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001, 107: 591-603. 10.1016/S0092-8674(01)00589-X.
Maurer MH, Grunewald S, Gassler N, Rossner M, Propst F, Wurz R, Weber D, Kuner T, Kuschinsky W, Schneider A: Cloning of a novel neuronally expressed orphan G-protein-coupled receptor which is up-regulated by erythropoietin, interacts with microtubule-associated protein 1b and colocalizes with the 5-hydroxytryptamine 2a receptor. J Neurochem. 2004, 91: 1007-1017. 10.1111/j.1471-4159.2004.02799.x.
Jimenez-Mateos EM, Wandosell F, Reiner O, Avila J, Gonzalez-Billault C: Binding of microtubule-associated protein 1B to LIS1 affects the interaction between dynein and LIS1. Biochem J. 2005, 389: 333-341. 10.1042/BJ20050244.
Seog DH: Glutamate receptor-interacting protein 1 protein binds to the microtubule-associated protein. Biosci Biotechnol Biochem. 2004, 68: 1808-1810. 10.1271/bbb.68.1808.
Seidenbecher CI, Landwehr M, Smalla KH, Kreutz M, Dieterich DC, Zuschratter W, Reissner C, Hammarback JA, Brockes TM, Gundelfinger ED, et al: Caldendrin but not calmodulin binds to light chain 3 of MAP1A/B: an association with the microtubule cytoskeleton highlighting exclusive binding partners for neuronal Ca(2+)-sensor proteins. J Mol Biol. 2004, 336: 957-970. 10.1016/j.jmb.2003.12.054.
Dallol A, Agathanggelou A, Fenton SL, Ahmed-Choudhury J, Hesson L, Vos MD, Clark GJ, Downwood J, Maher E, Latif E: RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res. 2004, 64: 4112-4116. 10.1158/0008-5472.CAN-04-0267.
Song MS, Chang JS, Song SJ, Yang TH, Lee H, Lim DS: The centrosomal protein RAS association domain family protein 1A (RASSF1A)-binding protein 1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J Biol Chem. 2005, 280: 3920-3927. 10.1074/jbc.M409115200.
Felsenstein J: PHYLIP: Phylogenetic Inference Package. 2002, Seattle: Department of Genetics, University of Washington, 3.6a
Acknowledgements
This work was supported by grants (to S.H.) from the National Institutes of Health. We thank Eric Hwang and Perihan Nalbant for helpful discussions and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Halpain, S., Dehmelt, L. The MAP1 family of microtubule-associated proteins. Genome Biol 7, 224 (2006). https://doi.org/10.1186/gb-2006-7-6-224
Published:
DOI: https://doi.org/10.1186/gb-2006-7-6-224