Abstract
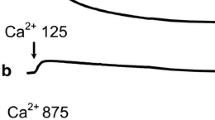
In liver mitochondria loaded with Ca2+ or Sr2+, α,ω-hexadecanedioic acid (HDA) can induce nonspecific permeability of the inner membrane (mitochondrial pore) by the mechanism insensitive to cyclosporin A (CsA). In this work we studied the effect of ionic strength of the incubation medium on the kinetics of the processes that accompany Ca2+-dependent induction of the mitochondrial pore by fatty acid: organelle swelling, Ca2+ release from the matrix, changes in transmembrane potential (Δψ) and rate of oxygen consumption, and the release of cytochrome c from the intermembrane space. Two basic incubation media were used: sucrose medium and isotonic ionic medium containing KCl without sucrose. We found that 200 μM Ca2+ and 20 μM HDA in the presence of CsA effectively induce high-amplitude swelling of mitochondria both in the case of sucrose and in the ionic incubation medium. In the presence of CsA, mitochondria can rapidly absorb Ca2+ and retain it in the matrix for a while without reducing Δψ. Upon incubation in the ionic medium, mitochondria retain most of the added Ca2+ in the matrix for a short time without reducing the Δψ. In both cases the addition of HDA to the mitochondria 2 min after the introduction of Ca2+ leads to the rapid release of these ions from the matrix and total drop in Δψ. The mitochondrial swelling induced by Ca2+ and HDA in non-ionic medium is accompanied by almost maximal stimulation of respiration. Under the same conditions, but during incubation of mitochondria in the ionic medium, it is necessary to add cytochrome c for significant stimulation of respiration. The mitochondrial swelling induced by Ca2+ and HDA leads to the release of cytochrome c in a larger amount in the case of ionic medium than for the sucrose medium. We conclude that high ionic strength of the incubation medium determines the massive release of cytochrome c from mitochondria and liberates it from the respiratory chain, which leads to blockade of electron transport along the respiratory chain and consequently to disruption of the energy functions of the organelles.
Similar content being viewed by others
Abbreviations
- CsA:
-
cyclosporin A
- HDA:
-
α,ω-hexadecanedioic acid
- TPP+ :
-
tetraphenylphosphonium
- Δψ:
-
transmembrane electrical potential
References
Skulachev, V. P., Bogachev, A. V., and Kasparinsky, F. O. (2010) Membrane Bioenergetics [in Russian], MSU, Moscow.
Malhi, H., Guicciardi, M. E., and Gores, G. J. (2010) Physiol. Rev., 90, 1165–1194.
Kroemer, G., Galluzzi, L., and Brenner, C. (2007) Physiol. Rev., 87, 99–163.
Zorov, D. B., Plotnikov, E. Y., Jankauskas, S. S., Isaev, N. K., Silachev, D. N., Zorova, L. D., Pevzner, I. B., Pulkova, N. V., Zorov, S. D., and Morosanova, M. A. (2012) Biochemistry (Moscow), 77, 742–753.
Skulachev, V. P. (2012) Biochemistry (Moscow), 77, 689–706.
Azzolin, L., von Stockum, S., Basso, E., Petronilli, V., Forte, M. A., and Bernardi, P. (2010) FEBS Lett., 584, 2504–2509.
Rasola, A., and Bernardi, P. (2011) Cell Calcium, 50, 222–233.
Crouser, E. D., Gadd, M. E., Julian, M. W., Huff, J. E., Broekemeier, K. M., Robbins, K. A., and Pfeiffer, D. R. (2003) Anal. Biochem., 317, 67–75.
Gilkerson, R. W., Selker, J. M., and Capaldi, R. A. (2003) FEBS Lett., 546, 355–358.
Petrosillo, G., Ruggiero, F. M., Pistolese, M., and Paradies, G. (2004) J. Biol. Chem., 279, 53103–53108.
Ott, M., Robertson, J. D., Gogvadze, V., Zhivotovsky, B., and Orrenius, S. (2002) Proc. Natl. Acad. Sci. USA, 99, 1259–1263.
Gogvadze, V., Orrenius, S., and Zhivotovsky, B. (2006) Biochim. Biophys. Acta, 1757, 639–647.
Krasnikov, B. F., Melik-Nubarov, N. S., Zorova, L. D., Kuzminova, A. E., Isaev, N. K., Cooper, A. J., and Zorov, D. B. (2011) Am. J. Physiol. Cell Physiol., 300, 1193–1203.
Schonfeld, P., and Bohnensack, R. (1997) FEBS Lett., 420, 167–170.
Bodrova, M. E., Dedukhova, V. I., Samartsev, V. N., and Mokhova, E. N. (2000) IUBMB Life, 50, 189–194.
Sultan, A., and Sokolove, P. (2001) Arch. Biochem. Biophys., 386, 37–51.
Sultan, A., and Sokolove, P. (2001) Arch. Biochem. Biophys., 386, 52–61.
Mironova, G. D., Gritsenko, E., Gateau-Roesch, O., Levrat, C., Agafonov, A., Belosludtsev, K., Prigent, A., Muntean, D., Dubois, M., and Ovize, M. (2004) J. Bioenerg. Biomembr., 36, 171–178.
Belosludtsev, K. N., Belosludtseva, N. V., and Mironova, G. D. (2005) Biochemistry (Moscow), 70, 815–821.
Sanders, R. J., Ofman, R., Valianpou, F., Kemp, S., and Wanders, R. J. (2005) J. Lipid Res., 46, 1001–1008.
Reddy, J. K., and Rao, M. S. (2006) Am. J. Physiol. Gastrointest. Liver Physiol., 290, G852–G858.
Wanders, R. J., Komen, J., and Kemp, S. (2011) FEBS J., 278, 182–194.
Tonsgard, J. H. (1986) J. Pediatr., 109, 440–445.
Kundu, R. K., Tonsgard, J. H., and Getz, G. S. (1991) J. Clin. Invest., 88, 1865–1872.
Orellana, M., Rodrigo, R., and Valdes, E. (1998) Gen. Pharmacol., 31, 817–820.
Dubinin, M. V., Adakeeva, S. I., and Samartsev, V. N. (2013) Biochemistry (Moscow), 78, 412–417.
Samartsev, V. N., Smirnov, A. V., Zeldi, I. P., Markova, O. V., Mokhova, E. N., and Skulachev, V. P. (1997) Biochim. Biophys. Acta, 1339, 251–257.
Kamo, N., Muratsugu, M., Hondoh, R., and Kobatake, Y. (1979) J. Membr. Biol., 49, 105–121.
Appaix, F., Minatchy, M., Riva-Lavieille, C., Olivares, J., Antonsson, B., and Saks, V. A. (2000) Biochim. Biophys. Acta, 1457, 175–181.
Wojtczak, L., and Schonfeld, P. (1993) Biochim. Biophys. Acta, 1183, 41–57.
Ichas, F., and Mazat, J.-P. (1998) Biochim. Biophys. Acta, 1366, 33–50.
Rybakova, S. R., Dubinin, M. V., and Samartsev, V. N. (2013) Biochemistry (Moscow), Suppl. Ser. A: Membr. Cell Biol., 7, 58–66.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2014, Vol. 79, No. 6, pp. 724–731.
Rights and permissions
About this article
Cite this article
Dubinin, M.V., Vedernikov, A.A., Khoroshavina, E.I. et al. Induction of Ca2+-dependent cyclosporin a-insensitive nonspecific permeability of the inner membrane of liver mitochondria and cytochrome c release by α,ω-hexadecanedioic acid in media of varying ionic strength. Biochemistry Moscow 79, 571–576 (2014). https://doi.org/10.1134/S000629791406011X
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629791406011X