Abstract
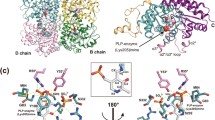
Crystal structures of Citrobacter freundii methionine γ-lyase complexes with the substrates of γ-(L-1-amino-3-methylthiopropylphosphinic acid) and β-(S-ethyl-L-cysteine) elimination reactions and the competitive inhibitor L-nor-leucine have been determined at 1.45, 1.8, and 1.63 Å resolution, respectively. All three amino acids occupy the active site of the enzyme but do not form a covalent bond with pyridoxal 5′-phosphate. Hydrophobic interactions between the active site residues and the side groups of the substrates and the inhibitor are supposed to cause noncovalent binding. Arg374 and Ser339 are involved in the binding of carboxyl groups of the substrates and the inhibitor. The hydroxyl of Tyr113 is a potential acceptor of a proton from the amino groups of the amino acids.
Similar content being viewed by others
Abbreviations
- Met-PH :
-
L-1-amino-3-methylthiopropylphosphinic acid
- MGL:
-
methionine γ-lyase
- PLP:
-
pyridoxal-5′-phosphate
References
Tanaka, H., Esaki, N., and Soda, K. (1985) Enzyme Microb. Technol., 7, 530–537.
Alferov, K. V., Faleev, N. G., Demidkina, T. V., Khurs, E. N., Morozova, E. A., and Khomutov, R. M. (2006) Dokl. Biokhim. Biofiz., 407, 102–105.
Dingwall, J. G. (1986) Abstr. III Int. Conf. Chem. and Biotech. Biol. Active Natural Products, Sofia, Bulgaria, Vol. 1, pp. 87–103.
Zhukov, Yu. N., Vavilova, N. A., Osipova, T. I., Voinova, T. M., Khurs, E. N., Dzhavakhiya, V. G., and Khomutov, R. M. (2004) Dokl. Akad. Nauk, 397, 120–123.
Khomutov, R. M., Zhukov, Yu. N., Khomutov, A. R., Khurs, E. N., Kramer, D. L., Miller, J. T., and Porter, C. W. (2000) Bioorg. Khim., 26, 718–720.
El-Sayed, Ashraf S. (2010) Appl. Microbiol. Biotechnol., 86, 445–467.
Manukhov, I. V., Mamaeva, D. V., Rastorguev, S. M., Faleev, N. G., Morozova, E. A., Demidkina, T. V., and Zavilgelsky, G. B. (2005) J. Bacteriol., 187, 3889–3893.
Ito, S., Nakamura, T., and Eguchi, Y. (1976) J. Biochem., 79, 1263–1272.
Nakayama, T., Esaki, N., Lee, W.-J., Tanaka, I., Tanaka, H., and Soda, K. (1984) Agric. Biol. Chem., 48, 2367–2369.
Kreis, W., and Hession, C. (1973) Cancer Res., 33, 1862–1865.
Yoshimura, M., Nakano, Y., Yamashita, Y., Oho, T., Saito, T., and Koga, T. (2000) Infect. Immunol., 68, 6912–6916.
Tokoro, M., Asai, T., Kobayashi, S., Takeuchi, T., and Nozaki, T. (2003) J. Biol. Chem., 278, 42717–42727.
Lockwood, B., and Coombs, G. (1991) Biochem. J., 279, 675–682.
Rebeille, F., Jabrin, S., Bligny, R., Loizeau, K., Gambonnet, B., van Wilder, V., Douce, R., and Ravanel, S. (2006) Proc. Natl. Acad. Sci. USA, 103, 15687–15692.
Coombs, G. H., and Mottram, J. C. (2001) Antimicrob. Agents Chemother., 45, 1743.
Yoshimura, M., Nakano, Y., and Koga, T. (2002) Biochem. Biophys. Res. Commun., 292, 964–968.
Sato, D., Kobayashi, S., Yasui, H., Shibata, N., Toru, T., Yamamoto, M., Tokoro, G., Ali, V., Soga, T., Takeuchi, T., Suematsu, M., and Nozaki, T. (2010) Int. J. Antimicrob. Agents, 35, 56–61.
Yoshioka, T., Wada, T., Uchida, N., Maki, H., Yoshida, H., Ide, N., Kasai, H., Hojo, K., Shono, K., Maekawa, R., Yagi, S., Hoffman, R. M., and Sugita, K. (1998) Cancer Res., 58, 2583–2587.
Miki, K., Xu, M., An, Z., Wang, X., Yang, M., Al-Refaie, W., Sun, X., Baranov, E., Tan, Y., Chishima, T., Shimada, H., Moossa, A. R., and Hoffman, R. M. (2000) Cancer Gene Ther., 7, 332–338.
Miki, K., Al-Refaie, W., Xu, M., Jiang, P., Tan, Y., Bouvet, M., Zhao, M., Gupta, A., Chishima, T., Shimada, H., Makuuchi, M., Moossa, A. R., and Hoffman, R. M. (2000) Cancer Res., 60, 2696–2702.
Tan, Y., Xu, M., and Hoffman, R. M. (2010) Anticancer Res., 30, 1041–1046.
Hoffman, R. M. (1997) Hum. Cell, 10, 69–80.
Hanniffy, S. B., Philo, M., Pelaez, C., Gasson, M. J., Requena, T., and Martinez-Cuesta, M. C. (2009) Appl. Environ. Microbiol., 75, 2326–2332.
Esaki, N., Suzuki, T., Tanaka, H., Soda, K., and Rando, R. R. (1977) FEBS Lett., 84, 309–312.
Esaki, N., Tanaka, H., Uemura, S., Suzuki, T., and Soda, K. (1979) Biochemistry, 18, 407–410.
Tanaka, H., Esaki, N., and Soda, K. (1977) Biochemistry, 16, 100–6.
Inoue, H., Inagaki, K., Adachi, N., Tamura, T., Esaki, N., Soda, K., and Tanaka, H. (2000) Biosci. Biotechnol. Biochem., 64, 2336–2343.
Takakura, T., Mitsushima, K., Yagi, S., Inagaki, K., Tanaka, H., Esaki, N., Soda, K., and Takimoto, A. (2004) Anal. Biochem., 327, 233–240.
McKie, A. E., Edlind, T., Walker, J., Mottram, J. C., and Coombs, G. H. (1998) J. Biol. Chem., 273, 5549–5556.
Yamagata, W., Kamei, K., Nozaki, T., Harada, S., and Sato, D. (2006) Acta Cryst., F62, 1034–1036.
Kudou, D., Misaki, S., Yamashita, M., Tamura, T., Takakura, T., Yoshioka, T., Yagi, S., Hoffman, R. M., Takimoto, A., Esaki, N., and Inagaki, K. (2007) J. Biochem. (Tokyo), 141, 535–544.
Nikulin, A., Revtovich, S., Morozova, E., Nevskaya, N., Nikonov, S., Garber, M., and Demidkina, T. (2008) Acta Cryst., D64, 211–218.
Bourenkov, G. P., and Popov, A. N. (2006) Acta Cryst., D62, 58–64.
Kabsch, W. (2010) Acta Cryst., D66, 125–132.
Adams, P. D., Grosse-Kunstleve, R. W., Hung, L.-W., Ioerger, T. R., McCoy, A. J., Moriarty, N. W., Read, R. J., Sacchettini, J. C., Sauter, N. K., and Terwilliger, T. C. (2002) Acta Cryst., D58, 1948–1954.
Emsley, P., and Cowtan, K. (2004) Acta Cryst., D60, 2126–2132.
Mamaeva, D. V., Morozova, E. A., Nikulin, A. D., Revtovich, S. V., Nikonov, S. V., Garber, M. B., and Demidkina, T. V. (2005) Acta Cryst., F61, 546–549.
Kack, H., Sandmark, J., Gibson, K., Schneider, H., and Lindqvist, Y. (1999) J. Mol. Biol., 291, 857–876.
Nakayama, T., Esaki, N., Sugie, K., Beresov, T. T., Tanaka, H., and Soda, K. (1984) Anal. Biochem., 138, 421–424.
Ronda, L., Bazhulina, N. P., Morozova, E. A., Revtovich, S. V., Chekhov, V. O., Nikulin, A. D., Demidkina, T. V., and Mozzarelli, A. (2010) Biochim. Biophys. Acta, Epub ahead of print.
Clausen, T., Huber, R., Laber, B., Pohlenz, H. D., and Messerschmidt, A. (1996) J. Mol. Biol., 262, 202–224.
Messerschmidt, A., Worbs, M., Steegborn, C., Wahl, M. C., Huber, R., Laber, B., and Clausen, T. (2003) Biol. Chem., 384, 373–386.
Faleev, N. G., Alferov, K. V., Tsvetikova, M. A., Morozova, E. A., Revtovich, S. V., Khurs, E. N., Vorob’ev, M. M., Phillips, R. S., Demidkina, T. V., and Khomutov, R. M. (2009) Biochim. Biophys. Acta, 1794, 1414–1420.
Morozova, E. A., Bazhulina, N. P., Anufriyeva, N. V., Mamayeva, D. V., Tkachev, Y. V., Streltsov, S. A., Timofeev, V. P., Faleev, N. G., and Demidkina, T. V. (2010) Biochemistry (Moscow), 75, 1272–1280.
Manukov, I. V., Mamayeva, D. V., Morozova, E. A., Rastorguyev, S. M., Faleev, N. G., Demidkina, T. Z., and Zavigelsky, G. B. (2006) Biochemistry (Moscow), 71, 361–369.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Original Russian Text © S. V. Revtovich, E. A. Morozova, E. N. Khurs, L. N. Zakomirdina, A. D. Nikulin, T. V. Demidkina, R. M. Khomutov, 2011, published in Biokhimiya, 2011, Vol. 76, No. 5, pp. 692–700.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM10-394, April 3, 2011.
Rights and permissions
About this article
Cite this article
Revtovich, S.V., Morozova, E.A., Khurs, E.N. et al. Three-dimensional structures of noncovalent complexes of Citrobacter freundii methionine γ-lyase with substrates. Biochemistry Moscow 76, 564–570 (2011). https://doi.org/10.1134/S0006297911050063
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297911050063