Abstract
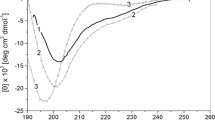
Equilibrium unfolding of stem bromelain (SB) with urea as a denaturant has been monitored as a function of pH using circular dichroism and fluorescence emission spectroscopy. Urea-induced denaturation studies at pH 4.5 showed that SB unfolds through a two-state mechanism and yields ΔG (free energy difference between the fully folded and unfolded forms) of ∼5.0 kcal/mol and C m (midpoint of the unfolding transition) of ∼6.5 M at 25°C. Very high concentration of urea (9.5 M) provides unusual stability to the protein with no more structural loss and transition to a completely unfolded state.
Similar content being viewed by others
Abbreviations
- CD:
-
circular dichroism
- D:
-
denatured state
- F:
-
folded state
- I:
-
intermediate state
- MRE:
-
mean residue ellipticity
- SB:
-
stem bromelain
References
Kim, P. S., and Baldwin, R. L. (1990) Ann. Rev. Biochem., 59, 613–660.
Reyna, A. A., and Arana, A. H. (1995) Biochim. Biophys. Acta, 1248, 123–128.
Jemmings, O. A., and Wright, P. E. (1993) Science, 262, 892–895.
Kuwajima, K. (1989) Proteins, 6, 87–103.
Ptitsyn, O. B. (1987) J. Protein Chem., 6, 273–293.
Sanz, J. M., and Gallego, G. G. (1997) Eur. J. Biochem., 240, 328–335.
Cohen, L. W., Coghlan, V. M., and Dihe, L. C. (1986) Gene, 48, 219–227.
Carne, A., and Moore, C. H. (1978) Biochem. J., 173, 73–83.
Dubois, T., Kleinschmidt, T., Schnek, A. G., Looze, Y., and Braunitzer, G. (1988) Biol. Chem. Hoppe-Seyler, 369, 741–754.
Topham, C. M., Salih, E., Frazao, C., Kowlessur, D., Overington, J. P., Thomas, M., Brocklehurst, S. M., Patel, M., Thomas, E. W., and Brocklehurst, K. (1991) Biochem. J., 280, 79–92.
Seyler, H. (1989) Biol. Chem., 370, 425–434.
Ritonja, A., Rowan, A. D., Buttle, D. J., Railings, N. D., Turk, V., and Barett, A. J. (1989) FEBS Lett., 247, 419–424.
Watson, D. C., Yaguchi, M., and Lynn, K. R. (1990) Biochem. J., 266, 75–81.
Kamphuis, I. G., Kalk, K. H., Swarte, M. B. A., and Drenth, J. (1984) J. Mol. Biol., 179, 233–257.
Baker, E. N. (1980) J. Mol. Biol., 141, 441–484.
Drenth, J., Iansonius, J. N., Koekoek, R., and Wolthers, B. G. (1971) Adv. Protein Chem., 25, 79–86.
Edwin, F., and Jagannadham, M. V. (2000) Biochim. Biophys. Acta, 1479, 69–82.
Edwin, F., and Jagannadham, M. V. (2002) Biochem. Biophys. Res. Commun., 290, 1441–1446.
Dubey, V. K., and Jagannadham, M. V. (2003) Biochemistry, 42, 12287–12297.
Dubey, V. K., Shah, A., Jagannadham, M. V., and Kayastha, A. M. (2006) Protein Pept. Lett., 6, 545–547.
Dubey, V. K., and Jagannadham, M. V. (2003) Phytochemistry, 62, 1057–1071.
Sundd, M., Kundu, S., Dubey, V. K., and Jagannadham, M. V. (2004) J. Biochem. Mol. Biol., 37, 586–596.
Thakurta, P. G., Biswas, S., Chakrabarti, C., Sundd, M., Jagannadham, M. V., and Dattagupta, J. K. (2004) Biochemistry, 43, 1532–1540.
Patel, B. K., and Jagannadham, M. V. (2003) J. Agric. Food Chem., 51, 6326–6334.
Sundd, M., Kundu, S., and Jagannadham, M. V. (2000) J. Protein Chem., 3, 169–176.
Kundu, S., Sundd, M., and Jagannadham, M. V. (1999) Biochem. Biophys. Res. Commun., 264, 635–642.
Murachi, T., and Yamazaki, M. (1970) Biochemistry, 9, 1935–1938.
Haq, S. K., Rasheedi, S., and Khan, R. H. (2002) Eur. J. Biochem., 269, 47–52.
Ahmad, B., and Khan, R. H. (2006) J. Biochem. (Tokyo), 140, 501–508.
Haq, S. K., Rasheedi, S., Sharma, P., Ahmad, B., and Khan, R. H. (2005) Int. J. Biochem. Cell Biol., 37, 361–374.
Gupta, P., Khan, R. H., and Saleemuddin, M. (2003) Arch. Biochem. Biophys., 413, 199–206.
Ahmad, B., Ansari, M. A., Sen, P., and Khan, R. H. (2006) Biopolymers, 81, 350–359.
Vanhoof, G., Cooreman, W., Lauwers, A., and Scharpe, S. (eds.) (1997) Bromelain in Pharmaceutical Enzymes, Marcel Dekker Inc., New York, pp. 131–147.
Sharpira, E., and Arnon, R. (1969) J. Biol. Chem., 244, 4989–4994.
Chen, Y. H., Yang, J. T., and Martinez, H. (1972) Biochemistry, 11, 4120–4131.
Pace, C. N. (1990) Trends Biotechnol., 8, 93–98.
Mucke, M., and Schmid, F. X. (1994) Biochemistry, 33, 12930–12935.
Soulages, J. S. (1998) Biophys. J., 75, 484–492.
Uversky, V. N., Karnoup, A. S., Seshadri, S., Doniach, S., and Fink, A. L. (1998) J. Mol. Biol., 278, 879–894.
Fink, A. L., Oberg, K. A., and Seshadri, S. (1998) Fold. Des., 3, 19–25.
Tanaka, N., Nishizawa, H., and Kunugi, S. (1997) Biochim. Biophys. Acta, 1338, 13–20.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2009, Vol. 74, No. 12, pp. 1642–1649.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM09-098, November 8, 2009.
Rights and permissions
About this article
Cite this article
Ahmad, B., Rathar, G.M., Varshney, A. et al. pH-dependent urea-induced unfolding of stem bromelain: Unusual stability against urea at neutral pH. Biochemistry Moscow 74, 1337–1343 (2009). https://doi.org/10.1134/S0006297909120062
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297909120062