Abstract
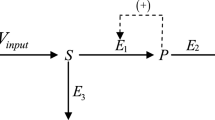
A steady-state approximation of the generalized two-dimensional model of a bifunctional enzyme catalyzing independent proceeding of two one-pathway reactions is considered in a case of mutual influence of the active sites. Coexistence of fast and slow catalytic cycles in the reaction mechanism is analyzed. Conditions when the hierarchy of fast and slow catalytic cycles allows simplification of a two-dimensional model and its reduction to the one-dimensional cyclic schemes were determined. Kinetic equations describing these simplified schemes are presented.
Similar content being viewed by others
References
Yourno, J., Kohno, T., and Roth, J. R. (1970) Nature, 228, 820–825.
Smith, S. (1994) FASEB J., 8, 1248–1259.
Liang, P. H., and Anderson, K. S. (1998) Biochemistry, 37, 12195–12205.
Huang, X., Holden, H. M., and Raushel, F. M. (2001) Annu Rev. Biochem., 70, 149–180.
Meek, T. D., Garvey, E. P., and Santi, D. V. (1985) Biochemistry, 24, 678–686.
Miles, E. W., Rhee, S., and Davies, D. R. (1999) J. Biol. Chem., 274, 12193–12196.
Trujillo, M., Donald, R. G. K., Roos, D. S., Greene, P. J., and Santi, D. V. (1996) Biochemistry, 35, 6366–6374.
Schneider, T. R., Gerhardt, E., Lee, M., Liang, P. H., Anderson, K. S., and Schlichting, I. (1998) Biochemistry, 37, 5394–5406.
Leys, D., Basran, J., and Scrutton, N. S. (2003) EMBO J., 22, 4038–4048.
Atreya, C. E., and Anderson, K. S. (2004) J. Biol. Chem., 279, 18314–18322.
Gehring, A. M., Lees, W. J., Mindiola, D. J., Walsh, C. T., and Brown, E. D. (1996) Biochemistry, 35, 579–585.
Miles, B. W., Banzon, J. A., and Raushel, F. M. (1998) Biochemistry, 37, 16773–16779.
Kim, J. H., Krahn, J. M., Tomchick, D. R., Smith, J. L., and Zalkin, H. (1996) J. Biol. Chem., 271, 15549–15557.
Johnson, E. F., Hinz, W., Atreya, C. E., Maley, F., and Anderson, K. S. (2002) J. Biol. Chem., 277, 43126–43136.
Eling, T. E., Glasgow, W. C., Curtis, J. F., Hubbard, W. C., and Handler, J. A. (1991) J. Biol. Chem., 266, 12348–12355.
Easterby, J. S. (1981) Biochem. J., 199, 155–161.
Easterby, J. S. (1984) Biochem. J., 219, 843–847.
Liang, P. H., and Anderson, K. S. (1998) Biochemistry, 37, 12206–12212.
Bakovic, M., and Dunford, H. B. (1994) Biochemistry, 33, 6475–6482.
Kulmacz, R. J., Pendleton, R. B., and Lands, W. E. M. (1994) J. Biol. Chem., 269, 5527–5536.
Vrzheshch, P. V. (1999) Biochemistry (Moscow), 64, 421–430.
Volkenshtein, M. V., and Magarshak, Yu. B. (1970) Biofizika, 15, 777–784.
Cornish-Bowden, E. (1979) The Basics of Enzymatic Kinetics [Russian translation], Mir, Moscow.
Cha, S. (1968) J. Biol. Chem., 243, 820–825.
Tikhonov, A. N. (1952) Mat. Sbornik, 31, 575–586.
Vrzheshch, P. V., and Varfolomeev, S. D. (1985) Biokhimiya, 50, 139–147.
Vrzheshch, P. V. (1988) Biokhimiya, 53, 1704–1711.
Vrzheshch, P. V. (1996) Biochemistry (Moscow), 60, 1481–1493.
Vrzheshch, P. V., Batanova, E. A., Mevkh, A. T., Varfolomeev, S. D., Gazaryan, I. G., and Thorneley, R. N. F. (2003) Biochem. J., 372, 713–724.
Vol’kenstein, M. V., and Gol’dstein, B. N. (1966) Biokhimiya, 31, 541–547.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © P.V. Vrzheshch, 2007, published in Biokhimiya, 2007, Vol. 72, No. 9, pp. 1153–1160.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM07-089, August 5, 2007.
Rights and permissions
About this article
Cite this article
Vrzheshch, P.V. Steady-state kinetics of bifunctional enzymes. Taking into account kinetic hierarchy of fast and slow catalytic cycles in a generalized model. Biochemistry Moscow 72, 936–943 (2007). https://doi.org/10.1134/S0006297907090039
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1134/S0006297907090039