Abstract
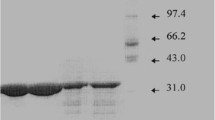
A preparative method for purification of a novel protease from the psychrotolerant Gram-negative microorganism Serratia proteamaculans (PSP) was developed using affinity chromatography on BPTI-Sepharose. It yielded electrophoretically homogeneous PSP preparation of 60 kD. The PSP properties (temperature and pH stability, high catalytic efficiency) indicate that this enzyme can be defined as a psychrophilic protease. Inhibitory analysis together with substrate specificity indicates that the studied PSP exhibits properties of serine trypsin-like and Zn-dependent protease.
Similar content being viewed by others
Abbreviations
- ATEE:
-
Nα-acetyl-L-tyrosine ethyl ester
- BAPNA:
-
Nα-benzoyl-DL-arginine p-nitroanilide
- BPTI:
-
bovine pancreatic trypsin inhibitor
- DFP:
-
diisopropylfluorophosphate
- DMSO:
-
dimethylsulfoxide
- DTDP:
-
4,4′-dithiodipyridine
- PMSF:
-
phenylmethylsulfonyl fluoride
- PSP:
-
Serratia proteamaculans protease
- STI:
-
soybean trypsin inhibitor
- TLCK:
-
Nα-p-tosyl-L-lysyl-chloromethyl-ketone
- Z-Lys-S-Bzl:
-
Nα-benzyloxycarbonyl-L-lysine thiobenzyl ester
- buffer A:
-
0.1 M Tris-HCl, pH 8.0, 50 mM CaCl2
- buffer B:
-
0.1 M Tris-HCl, pH 8.0
- buffer C:
-
0.1 M Tris-HCl, pH 9.0, 50 mM CaCl2, 1 mM MgCl2
- buffer D:
-
10 mM Hepes-KOH, pH 7.5, 1 mM MgCl2
References
Cavicchioli, R., Siddiqui, K. S., Andrews, D., and Sowers, K. R. (2002) Curr. Opin. Biotechnol., 13, 253–261.
Feller, G. (2003) Cell. Mol. Life Sci., 60, 648–662.
Kumar, S., Tsai, C. J., and Nussinov, R. (2002) Biochemistry, 41, 5359–5374.
Wintrode, P. L., and Arnold, F. H. (2000) Adv. Protein Chem., 55, 161–225.
Demidyuk, I. V., Kalashnikov, A. E., Gromova, T. Yu., Gasanov, E. V., Safina, D. R., Zabolotskaya, M. V., Rudenskaya, G. N., and Kostrov, S. V. (2006) Protein Exp. Purif., in press.
March, S. C., Parikh, I., and Cuatrecasas, P. (1974) Analyt. Biochem., 60, 149–152.
Walsh, K. A., and Wilcox, P. E. (1970) Meth. Enzymol., 19, 31–41.
Taran, L. D., and Smovdyr, I. N. (1992) Biokhimiya, 57, 55–60.
Asgersson, B., Fox, J. W., and Bjarnason, J. B. (1989) Eur. J. Biochem., 180, 85–94.
Genicot, S., Feller, G., and Gerday, Ch. (1988) Comp. Biochem. Physiol., 90B, 601–609.
Simpson, B. K., and Haard, N. F. (1984) Comp. Biochem. Physiol., 79B, 613–622.
Nakon, R., and Krishnamoorthy, C. R. (1983) Science, 221, 749–750.
Salamone, P. R., and Wodzinski, R. J. (1997) Appl. Microbiol. Biotechnol., 48, 317–321.
Wang, Q.-F., Miao, J.-L., Hou, Y., Ding, Yu., Wang, G.-D., and Li, G.-Y. (2005) Biotechnol. Lett., 27, 1195–1198.
Yoshida, N., Sasaki, A., and Inoue, H. (1971) FEBS Lett., 15, 129–132.
Kim, I. S., and Lee, K. J. (1996) Microbiology, 142, 1797–1806.
Baratty, J., and Maroux, S. (1976) Biochim. Biophys. Acta, 452, 488–496.
Mikhailova, A. G., Rumsh, L. D., Dalgalarrondo, M., Chobert, J. M., and Haertle, T. (2003) Biochemistry (Moscow), 68, 926–933.
Mikhailova, A. G., Likhareva, V. V., Vaskovsky, B. V., Garanin, S. K., Onoprienko, L. V., Prudchenko, I. A., Chikin, L. D., and Rumsh, L. D. (2004) Biochemistry (Moscow), 69, 909–917.
Zamolodchikova, T. S., Sokolova, E. A., Alexandrov, S. L., Mikhaleva, I. I., Prudchenko, I. A., Morozov, I. A., Kononenko, N. V., Mirgorodskaya, O. A., Da, U., Larionova, N. I., Pozdnev, V. F., Ghosh, D., Duax, W. L., and Vorotyntseva, T. I. (1997) Eur. J. Biochem., 249, 612–621.
Hamada, K., Hata, Y., Katsuya, Y., Hiramatsu, H., Fujiwara, T., and Katsube, Y. (1996) J. Biochem. (Tokyo), 119, 844–851.
Baumann, U. (1994) J. Mol. Biol., 42, 244–251.
Maeda, H., and Morihara, K. (1995) Meth. Enzymol., 248, 395–413.
Park, H., and Ming, L.-J. (2002) J. Biol. Inorg. Chem., 7, 600–610.
Mikhailova, A. G., Vorotyntseva, T. I., Bessmertnaya, L. Ya., and Antonov, V. K. (1984) Biokhimiya, 49, 1733–1738.
Rawlings, N. D., and Barrett, A. J. (2000) Nucleic Acids Res., 28, 323–325.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A. G. Mikhailova, V. V. Likhareva, R. F. Khairullin, N. L. Lubenets, L. D. Rumsh, I. V. Demidyuk, S. V. Kostrov, 2006, published in Biokhimiya, 2006, Vol. 71, No. 5, pp. 697–706.
Rights and permissions
About this article
Cite this article
Mikhailova, A.G., Likhareva, V.V., Khairullin, R.F. et al. Psychrophilic trypsin-type protease from Serratia proteamaculans . Biochemistry (Moscow) 71, 563–570 (2006). https://doi.org/10.1134/S0006297906050166
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1134/S0006297906050166