Abstract
Biocatalysts with lipase activity (BLAs) were prepared by adsorptive immobilization of recombinant lipase (rPichia/lip) from thermophilic microscopic fungi Thermomyces lanuginosus produced by a genetically engineered strain of methylotrophic yeast Komagataella phafii (Pichia pastoris). Supports with different physicochemical properties were used as adsorbents: mesoporous hydrophilic silica (SiO2) and macroporous hydrophobic carbon aerogel (MCA). The enzymatic activity, substrate specificity and operational stability of BLAs were studied in the esterification of saturated fatty acids with aliphatic alcohols differing in the number of carbon atoms in the molecule from 2 to 18. Matrices of relative activities were compiled for more than 60 pairs of substrates, an acid and an alcohol, by comparing the reaction rates of the esterification under identical conditions, which allowed us to reveal differences in the specificity of adsorbed lipase depending on the chemical nature of the support. It was found that for both types of biocatalysts, rPichia/lip on SiO2 (PLSi) and rPichia/lip on MCA (PLC), the maximum reaction rate was observed under esterification of heptanoic acid (C7) with butyl alcohol (C4). Under the same conditions of the synthesis of esters (20 ± 2°C, 1 bar, a mixture of hexane and diethyl ether as an organic solvent), including the synthesis of butylheptanoate, rPichia/lip adsorbed on silica showed an order of magnitude lower activity than lipase adsorbed on carbon aerogel. The catalytic constants, equal to 3.7 s–1 and 1.1 × 102 s–1, respectively, differed by 30 times. It was found that esters of short chain fatty acids C4–C7 and ethyl alcohol C2 were synthesized 2–3 times faster using the hydrophobic PLC type than using the hydrophilic PLSi type of BLAs. At the same time, esters of high-molecular-weight acids С9, C10, С18 and alcohols С8–С16 with pronounced hydrophobicity were synthesized 1.5–2 times faster using of PLSi type BLAs. The operational stability of the biocatalysts was quite high: the prepared BLAs retained 82–99% of their initial activity after more than 30 reaction cycles, while the duration of each cycle to reach an acid conversion above 85% was several hours (4–6 h).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The functional properties of immobilized enzymes, such as catalytic activity, substrate specificity, and stability, are shown to be determined by the physicochemical properties of the adsorbents used to immobilize them, such as surface texture and polarity/hydrophobicity. In recent decades, a research area called enzyme engineering by selecting a method of immobilization, which studies the changes (modulation) of the functional properties of immobilized enzymes, has been intensively developed. According to the authors of the review [1], enzyme engineering is fully compatible with other chemical and/or biological approaches used to improve functional properties of enzymes, and the success of this engineering work is determined by the availability of a wide range of immobilization protocols. Undoubtedly, the modulation of the functional properties of enzymes using enzyme engineering is an interesting and promising direction in the development of heterogeneous biocatalysis.
In their early works, the authors, in summarizing numerous results on the adsorptive immobilization of oxidoreductases (lactate and alcohol dehydrogenases, glucose oxidase, tyrosinase) on pyrocarbon-containing inorganic supports, including mesoporous θ-alumina, concluded that the mutual geometric and chemical correspondence of the physicochemical properties of the enzyme and the adsorbent surface was a necessary condition for the preparation of active and stable biocatalysts [2]. For example, geometric correspondence implied correspondence between pore sizes that predominate in the adsorbent texture and the size of the hydrated enzyme molecule (10 nm on average). This meant that mesopores larger than 10–15 nm were optimal: in such pores, the enzyme interacted with the pore walls at multiple points, its conformation became more rigid, and, as a result, its stability increased. Chemical correspondence implied, first of all, the presence of an optimal hydrophilic–hydrophobic balance between the properties of the enzyme and the surface of the adsorbent. For example, for glucose oxidase, the maximum stabilizing effect was observed under adsorption of this enzyme on mesoporous θ-Al2O3 containing 7–15 wt % pyrocarbon. The surface of such an adsorbent resembled a chessboard: black clusters of hydrophobic pyrocarbon formed on strong acid–base centers of alumina oxide and blocked them. The remaining areas of noncarbonized white Al2O3 had slightly acidic, polar (hydrophilic) properties [2]. On the other hand, upon adsorption of alcohol dehydrogenase and tyrosinase on these supports almost complete inactivation of these enzymes was observed; optimal supports contained 0.5–3.0 wt % carbon [2]. The problem of selecting the optimal adsorbent was solved individually for each definite enzyme.
Lipases (glycerol ester hydrolases, EC 3.1.1.3) catalyze a variety of reactions, both direct (hydrolysis of triglycerides) and reverse (ester synthesis), with the latter occurring in nonaqueous media where the water content does not exceed 1 vol %. Biocatalysts with lipase activity (BLAs) in both homogeneous (soluble) and heterogeneous (immobilized) states are widely used in various industries, such as:
1. Production of smart washing powders that remove oil and grease stains by enzymatic hydrolysis of triglycerides;
2. Manufacture of methyl/ethyl esters of fatty acids (FAs), which are part of the triglycerides of vegetable oils and cooking wastes, for the production of biodiesel as an additive to motor fuel by enzymatic alcoholysis (methanolysis, less often ethanolysis) of triglycerides [3–5];
3. Large scale manufacture of valuable food ingredients for the production of spreads and margarines, substitutes of cocoa butter and milk fats that do not contain unwanted trans-isomers of FAs and have the desired organoleptic and physicochemical properties: melting point, plasticity, consistency, creamy taste, by enzymatic interesterification oil-fat blends at increased temperatures (60–80°С) [6–9];
4. Synthesis of valuable esters by enzymatic esterification under very mild conditions (20–50°C, 1 bar).
It is known that esters are in great demand in the market of flavors, softening and moisturizing ingredients (emollients), surfactants, and emulsifiers in the food and cosmetic industries. Biocatalysts prepared by immobilization of lipases are intensively studied for performing green processes in organic chemistry [9–12].
The ability to control and modulate functional properties of lipases using enzyme engineering, namely, by selecting a support for immobilization, can be demonstrated by several examples. The catalytic properties of Candida antarctica lipase B in the reaction of hydrolytic separation of R- and S-isomers of (±)-2-O-butyryl-2-phenylacetic acid were changed (modulated) by immobilization on hydrophobic supports, such as butyl-(C4)-agarose, octyl-(C8)-agarose, and octadecyl-(C18)-Sepabeads [13]. With an increase in the hydrophobicity of the modifying fragments (C4 → C18), the reaction rate increased by 2 times, and the stereospecificity of biocatalysis also changed [13].
In [14], the authors studied the properties of Penicillium sp. lipase immobilized on the supports described above. For this enzyme, it was also found that with an increase in the hydrophobicity of the modifying fragments, the initial rate of p-nitrophenyl-(C16)-palmitate hydrolysis in a buffer solution and sardine oil hydrolysis in a two phase system with cyclohexane increased by many times (by 4.1 and 2.3 times, respectively) [14]. The authors suggested that supports that differ in hydrophobicity affect the conformation of the active site of the lipase and, as a result, provide enzyme hyperactivation and all the observed differences in the functional properties of the immobilized enzyme. In [15], it was shown that the rate of oil ethanolysis and the regioselectivity in a completely anhydrous medium depended on the nature of the supports used to immobilize T. lanuginosus lipase. Thus, when using C18-Sepabeads and C18-Purolite, nonselective biocatalysts were prepared, while when using supports modified with divinylbenzene groups, 1,3-selectivity toward triglycerides was observed [15].
Computer modeling (CM) of the 3D structure of enzyme molecules, including TLL (Thermomyces Lanuginosus Lipase), along with calculation and prediction of the effect of mutations introduced into the protein primary structure on the functional properties of enzymes, first of all, on thermal stability is a modern trend in biocatalysis [16]. The subsequent genetic engineering design of producer strains and the production of recombinant enzymes with altered functional properties is a logical continuation of such studies. Obviously, experimental techniques with modern CM methods allow solving not only practical, but also fundamental problems, that is, to reveal mechanisms of the process of enzyme immobilization on solid supports and to conduct a deep analysis of possible conformational rearrangements in the enzyme molecule during its immobilization, as well as the structural and functional features of the enzyme–support complex.
The authors of this article previously studied BLAs prepared by adsorption of recombinant lipase rPichia/lip on inorganic supports, in particular various carbon nanotubes (CNTs) [17] and also studied the processes of enzymatic low temperature synthesis of esters using the prepared biocatalysts, including the selection of an organic solvent for the reaction medium [18–23]. In this work, the authors continued the systematic study of BLAs and FAs esterification, analyzed all the results, and supplemented the research with modern computer simulation to analyze the structural and functional features of the lipase–support complex.
The aim of this work was to continue comparative studies of the functional properties of recombinant T. lanuginosus lipase (activity, substrate specificity, and stability) in the reaction of high-molecular-weight ester synthesis depending on the chemical nature of inorganic adsorbents, and to analyze the results in order to search for correlation dependences and immobilization regularities. In order to elucidate the mechanism of the TLL molecule interaction with a carbon nanotube (CNT), computer modeling using rigid and flexible molecular docking was performed.
MATERIALS AND METHODS
The recombinant T. lanuginosus lipase (designated as rPichia/lip) was produced by the methylotrophic yeast strain Pichia pastoris X-33, specially designed using genetic engineering manipulations, as described in [18]. BLAs were prepared using rPichia/lip buffer solutions (0.02 M phosphate buffer, pH 7.0) with a protein concentration of 2–15 mg mL–1.
The protein concentration in the solution was measured by the method [24] using the Coomassie G-250 dye (Sigma, United States). Solutions of bovine serum albumin (Sigma, United States) were used to construct a calibration curve.
The commercial silica (SiO2) trademark KSK-G (Karpov Chemical Plant, Russia) had the following textural parameters: specific surface (SspBET) 160 m2 g–1, pore volume (VΣ) 0.76 cm3 g–1.The porosity was 58%, mesopores 20 nm in diameter predominated in the porous structure of the silica. The size of SiO2 granules taken for the preparation of BLAs was 0.5–2 mm. A macroporous carbon aerogel (MCA) was obtained as a result of high-temperature pyrolysis of ethylene on a Fe:Co/CaCO3 catalyst [25, 26]. The bulk density of aerogel was 0.06 g cm–3. MCA granules were light black balls formed as a result of chaotic interlacing of CNTs [19, 25]. MCA had the following textural parameters: SspBET 100 ± 20 m2 g–1; VΣ 12 ± 3 cm3 g–1; the porosity was 98%. Macropores with a diameter of more than 1 μm predominated in the porous structure of MCA; the volume of mesopores with a diameter of 2–20 nm did not exceed 0.2% of VΣ. MCA granules of 3–4 mm in diameter were used to prepare BLAs. The textural parameters of the adsorbents were measured using nitrogen and mercury porosimetry on AutoPore 9200 and ASAP 2400 V3.07 equipment (Micromeritics Instrument Corporation, United States). The electron microscopic studies of original MCA and PLC type biocatalysts were performed using a JSM 6460 LV scanning electron microscope (SEM) (JEOL, Japan) and a JSM 2010 high resolution transmission electron microscope (TEM) (JEOL, Japan).
All reagents, including lipase substrates (fatty acids and alcohols), organic solvents (hexane, diethyl ether) were reagents made in Russia. The starting reagents and reaction products were analyzed by gas chromatography–mass spectrometry (GC–MS) on an Agilent 7000B GC/MS instrument (Agilent, United States) [23]. For all analyses, a highly polar column based on N-propyl-6-methyl-quinolinium-bis(trifluoromethylsulfonyl)imide ionic liquid (10 m × 0.25 mm × 0.2 μm) was used. The conditions of chromatographic analysis were as follows: first, the columns were kept for 3 min at 100°C, then the temperature was raised to 280°C at a rate of 10°C min–1; the temperature of the evaporator was also 280°С. The flow rate of the carrier gas (helium) was 1 mL min–1. The mass spectrometer operating conditions were electron ionization 70 eV, ionization source temperature 230°С, transition line temperature 250°С. The spectrum was recorded in the scanning mode in the range of 40–450 m/z.
Biocatalysts with lipase activity named as rPichia/lip on silica (PLSi) and rPichia/lip on carbon aerogel (PLC) were obtained by adsorptive immobilization of the recombinant lipase on appropriate supports. To prepare biocatalysts of the PLSi type, silica granules were preliminarily dried at 115°C for 4 h. Dried granules (1.0 g) were impregnated according to their moisture capacity with a lipase solution in buffer (0.02 M phosphate buffer, pH 7.0) with a protein concentration of 10 ± 4 mg mL–1, placed in a tightly closed vial, and were kept there for 5 h at room temperature (20 ± 2°C). The granules impregnated with a lipase solution were dried for 1–2 days under ambient conditions to a dry-air state. The amount of immobilized lipase (in mg g–1) was calculated taking into account the protein concentration and the volume of the impregnating solution equal to VΣ of silica (0.8 cm3 g–1).
PLC type biocatalysts were prepared as follows. MCA granules of 3–4 mm were placed into a lipase solution in buffer (0.02 M phosphate buffer, pH 7.0) with a protein concentration of 2 ± 1 mg mL–1, in the ratio of adsorbent weight : vol. solution = 1 : 100 and kept for 1 day at 20 ± 2°C with occasional stirring. The lipase solution was decanted, the biocatalysts were repeatedly washed with buffer, placed on a paper filter to remove excess water, and dried for 1–2 days under ambient conditions to dry-air state. The adsorption value (in mg g–1) was calculated taking into account the protein concentration before and after adsorption.
The prepared BLAs were studied in the esterification reaction of various pairs of substrates, saturated monocarboxylic acid (S1) and primary aliphatic alcohol (S2), differing in the length of the carbon skeleton with the number of carbon atoms from 2 to 18. Esterification of substrates (more than 60 pairs) was carried out under the following conditions: 22 ± 2°С; 1 bar; the initial concentrations of S1 and S2 were 0.25 ± 0.03 mol L–1 and 0.50 ± 0.04 mol L–1, respectively; a solvent was a mixture of hexane and diethyl ether in a ratio of 1 : 1 (vol/vol). Weighed samples of biocatalysts were poured with a solution of S1 and the initial concentration of the acid was determined; S2 was then added to the reaction medium in a two-fold molar excess. The content of biocatalysts in the reaction medium was 20.8 wt % for PLSi type and 0.8 wt % for PLC type biocatalysts. The initial reaction rate was determined from the linear section of the kinetic curve of the loss of a weak organic acid (S1), the concentration of which was analyzed by titrimetry using an ethanol solution of NaOH with a known molarity (0.0256 ± 0.0006 mol L–1) using phenolphthalein as an indicator of the equivalence point. The reaction rate and enzymatic activity of the biocatalysts were calculated and expressed, respectively, in µmol L–1 s–1 and activity units (U) per 1 g of dry biocatalyst (1 U = µmol min–1). The results of titrimetric and chromatographic analyzes coincided within the experimental error of 10%.
The activity of biocatalysts was measured in periodic reaction cycles, the duration of each cycle was determined by the time it took to reach an acid conversion of at least 85%. In the first cycle, the biocatalysts passed through the stage of conditioning; their activity increased by 2–4 times. This was due to the fact that water formed during esterification was accumulated inside the biocatalyst and a favorable aqueous microenvironment was formed for the adsorbed lipase, which retained the structure of enzyme molecules in a functional hydrated form. After the completion of esterification, the reaction mixture containing the ester, as well as a small amount of substrates S1 and S2, was decanted. The biocatalysts were repeatedly washed with a solvent, a mixture of hexane and diethyl ether, and then additionally kept in the same solvent for at least 20 h. The next reaction cycle was carried out with the washed biocatalyst under the conditions described above.
Statistical processing of the results was performed according to the Student’s t test with a confidence probability of 0.95; the number of measurements n = 3–6. The relative error did not exceed 10% (minimum 6%).
To calculate the geometry of lipase substrate molecules S1 and S2, the method of molecular mechanics (MM+ Force Field) in the HyperChem 7 software package (Hypercube, Inc.) was used.
Computer simulation of the interaction of the TLL molecule with a single-walled carbon nanotube was carried out using molecular docking. The crystal structure of TLL with a resolution of 2.3 Å was taken from the PDB database (PDB ID: 4ZGB). For simulation, a form of lipase in which the active site was in the closed conformation, was used [27]. CNT structures of various sizes were generated using the Nanotube Modeler program and optimized using Avogadro.
Molecular docking of lipase monomer and dimer with nanotubes of various lengths and diameters was carried out using the AutoDock Vina 1.1.2 software package, which allows flexible docking taking into account the mobility of side amino acid residues, in particular, GLU1A, LEU6A, LYS24A, LYS24B, ASN26B, PRO29A, ARG84A, ARG84B, PRO136A, ASP137A, LYS237A. These side residues of the lipase were considered as flexible after rigid docking, during which the most probable binding sites of CNT with the lipase were evaluated. The size of the given box was: x = 126; y = 118; z = 126; the lipase was placed into it. The spatial center of the lipase was located at the point with coordinates: x = 20.489; y = 49.005; z = 0.215. The calculation was reduced to automatic enumeration of spatial conformations and orientations of nanotubes in the binding site of the lipase in order to obtain the lipase–CNT complex with the minimum free energy of the system. The visualization of the simulation results was carried out in the MGLTools and PyMol programs.
RESULTS AND DISCUSSION
Studies of BLAs by scanning electron microscopy showed that the surface morphology of the original MCA and PLC type biocatalyst were almost the same: randomly intertwined CNTs were clearly observed, similarly to that described in [19]. After adsorption of the lipase, the openwork of the 3D structure of the carbon aerogel decreased; the interlacing of CNTs, which were thicker than those of the original support, became denser; smooth films were formed, probably of protein origin. As can be seen from the transmission electron microscope (TEM) images, amorphous deposits were observed at the junctions of the interlacing CNTs, apparently formed by the adsorbed rPichia/lip (Fig. 1). PLC type biocatalyst granules after drying decreased in diameter by 1.5–2 times compared with the original MCA granules obtained in a catalytic setup for high-temperature ethylene pyrolysis. After conducting the first two or three reaction cycles of esterification, BLA granules swelled due to the formation and accumulation of water formed during the reaction inside these biocatalysts. Considering the textural parameters of the support, silica and carbon aerogel, as well as the size of the hydrated lipase molecule, the surface area accessible for rPichia/lip adsorption was calculated taking the fact into account that pores with a diameter of 20 nm and more were accessible for immobilization. The accessible surface area (Saccess) of silica, equal to 77 m2 g–1, was 51% of SspBET. For macroporous carbon aerogel, as can be seen from the textural parameters, the entire surface of SspBET, equal to 100 m2 g–1, is accessible for lipase adsorption. Despite similar Saccess values, the amounts of adsorbed lipase on the studied supports differed by an order of magnitude. PLSi type biocatalysts contained 6.2–14.0 mg g–1 (the amount of mg of protein per 1 g of support). The adsorption value of rPichia/lip on MCA was 100 ± 20 mg g–1.
The catalytic properties of the immobilized lipase calculated from the complete Michaelis–Menten kinetic curve using the Origin® software (OriginLab Corp., United States), such as maximum reaction rate, Michaelis constants, catalytic constants (k, s–1) depended on the chemical nature of the support: for lipase immobilized on silica, the catalytic constant was ~30 times lower than for rPichia/lip adsorbed on MCA, namely, the k values were 3.7 s–1 and 1.1 × 102 s–1, respectively. The maximum activity of the prepared BLAs, as measured in the esterification of heptanoic acid with n-butanol, was 22.3 ± 2.0 and 513.9 ± 44.4 U g–1 for PLSi and PLC types biocatalysts, respectively [18].
The results of a comprehensive study of the substrate specificity of the adsorbed rPichia/lip depending on the chemical nature of the support, hydrophilic silica and hydrophobic carbon aerogel, were presented in the form of relative activity (Arel) matrices in [20, 21], as well as in the present work, in which the substrate specificity of PLC type biocatalysts toward high-molecular-weight substrates S1 (C7 and more) was the most detailed and fully studied. It was shown that both types of BLAs were characterized by broad substrate specificity in the synthesis of a wide range of esters of saturated monocarboxylic (fatty) acids and primary aliphatic alcohols under very mild conditions (20 ± 2°C, 1 bar); acid conversion not lower than 85% was achieved within 2–4 h.
Comparative analysis of the results also showed that the following properties were common for two types of BLAs: (1) the rate of the synthesis of heptanoic acid ester with n- or iso-butanol (butylheptanoate) was maximum and Arel was taken as 1.0 in calculations; (2) in most cases, the rate of the synthesis of ethyl esters (S2 was ethanol, C2) was minimal compared with the rate of esterification with the participation of higher-molecular-weight alcohols, such as propanol C3 and butanol C4; (3) the rate of the synthesis of esters of iso-C4–C5 acid isomers was 1–2 orders of magnitude lower compared with their linear molecules, while the isomerism of C4–C5 alcohols had almost no effect on the esterification rate [22]; and (4) the rate of the esterification of secondary alcohols was an order of magnitude lower compared with primary ones; tertiary alcohols did not participate in the esterification reaction [20, 21].
Overall comparative studies of two types of prepared biocatalysts using a wide range of S1 and S2 substrate pairs (more than 60) allowed us to obtain numerous experimental results, analyze them, and determine how the chemical nature of the supports changed (modulated) the substrate specificity of the immobilized recombinant lipase rPichia/lip. Since the immobilized lipase was more sensitive to the S1 substrate, the fatty acids C7, C9, C10, C12, C18 were considered individually, and S2 substrates (alcohols) were divided into three groups depending on their polarity, which was characterized by logP [28]: group 1 (logP < 1) included C2 (ethanol), C3 (propanol), C4 (butanol); group 2 (1 < logP < 4) included C5 (pentanol), C8 (octanol); group 3 (logP > 4) included C10 (decanol), C11 (undecanol), C12 (dodecanol), C16 (hexadecanol).
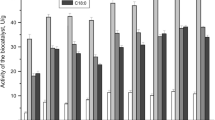
The observed differences in the substrate specificity of BLAs depending on the chemical nature (hydrophilicity/hydrophobicity) of the adsorbents used for adsorptive immobilization of rPichia/lip are shown in Fig. 2, where grey columns denoted PLSi type biocatalyst and black columns denoted PLC type. Comparison of the heights of the columns corresponding to Arel allowed us to find the following. Significant differences between biocatalysts of different types were observed in the synthesis of ethyl esters of FAs of various lengths (S2 was ethanol, С2Н5ОН): the rates of esterification were significantly higher for biocatalysts of the PLC type: by 2.4–2.8 times for C4–6 acids [21] and by 1.2–1.6 times for C7–18 acids (Fig. 2). It is known that ethanol can inactivate various enzymes, including immobilized rPichia/lip [29]. Since the hydrophobicity of the original MCA in the presence of ethanol significantly decreased due to adsorption of ethanol molecules on CNTs, this adsorbent was apparently capable of exhibiting a protective effect by reducing the local concentration of C2H5OH in the lipase microenvironment. For another alcohol from group 1, butanol, such a protective effect was not observed. When comparing the values of Arel, for example, in the reaction of the synthesis of butyl esters of heptanoic С7 (Fig. 2a) and stearic С18 acids (Fig. 2d), it turned out that the relative activity of BLAs of the PLSi and PLC types decreased by 1.3 (for С7) and 3.5 (for C18). If S2 belonged to group 2, the relative rate of esterification of C7–18 acids using PLC type was also significantly lower (1.5–2 times) (Fig. 2), which was a very unexpected result. If S2 belonged to group 3, then, in most cases, no special differences in the relative rates of ester synthesis were observed (Fig. 2), which was also an unobvious fact. It is well known that hydrophobic high-molecular-weight substrates S1 and S2 have a higher affinity to the carbon surface of CNTs compared with SiO2, thereby their surface concentration increased due to hydrophobic interactions with the surface, which can lead to an increase in the rate of their esterification. However, the same interactions can reduce the local concentration of substrates near the immobilized lipase, or hinder breakdown of the enzyme–substrate complex, or prevent desorption of esters as a higher-molecular-weight and more hydrophobic reaction product compared with S1 and S2. As a result, the rate of the esterification process as a whole did not increase. Thus, hydrophilic PLSi type were more efficient biocatalysts for the synthesis of high-molecular-weight esters compared with hydrophobic PLC type.
The relative activity (U) of PLSi type (grey columns) and PLC type (black columns) biocatalysts and in the esterification reaction of saturated fatty acids: a, heptanoic (enanthic, C7); b, nonanoic (pelargonic, C9); c, decanoic (capric, C10); d, octadecanoic (stearic, C18) by aliphatic alcohols with different lengths of the carbon skeleton (n is the number of carbon atoms).
In the present work, attempts to find correlations between the esterifying activity of BLAs and physicochemical characteristics of lipase substrates were made. As seen in Fig. 3a, the polarity of aliphatic alcohols (S2), as characterized by the value of logP, increased linearly with the elongation of the carbon chain of their molecules. At the same time, no monotonic dependence or correlation of the biocatalytical activity on the polarity of S2 was observed for the studied С7, С9, and С12 acids (S1) (Fig. 3b). As a result of repeated experiments, it was found that the rate of synthesis of butanol C4 esters for all the studied acids was at its maximum, and that of decanol C10 esters was reproducibly minimum (Fig. 3b). It was also shown that decanol (decyl alcohol, С10) had a negative effect on both the activity and stability of PLSi type biocatalysts: for example, during seven reaction cycles of esterification of heptanoic acid С7 with decanol, the initial activity of PLSi type decreased on average by 3 times (Fig. 4a), while the activity of the PLC type biocatalyst decreased by 1.4 times over six reaction cycles (Fig. 4b). Biocatalysts of both types showed high activities and stability in the butylheptanoate synthesis reaction (Fig. 4).
The polarity (logP) of alcohol substrates S2 depending on the number of carbon atoms in the molecule (a), dependence of the PLC type biocatalyst activity on logP parameter (b) in the reaction of ester synthesis of: 1, nonanoic (pelargonic, C9); 2, dodecanoic (lauric, C12); 3, heptanoic (enanthic, C7) acids.
The pronounced negative effect of decanol (S2) on the functional properties of the immobilized rPichia/lip did not depend on either the nature of the acid substrate (S1) or on the physicochemical properties of the adsorbents: for both types of BLAs, similar dependences were observed, for example, minima in the rates of the esterification reaction with the participation of decanol C10 (Fig. 3b). The deepest minimum was characteristic of the esterification of dodecanoic acid (lauric, С12) with decanol С10: for example, the esterifying activity of BLA in the reaction of the synthesis of decyl dodecanoate (Fig. 3b, 2) was 3.2 times lower compared with the rate of the synthesis of butyl esters of this acid. For enanthic (heptanoic, C7) and nonanoic (pelargonic, C9) acids (Fig. 3b, 1 and 3), the difference in the rates of the synthesis of decyl esters of these acids was 2.2 times. It seemed interesting that the addition of one CH2-group to the decanol molecule leads to a 2–4-fold increase in the activity of the biocatalyst (Fig. 3b). These data can be explained by the topology of the active site of the enzyme (E), as well as the processes of formation and breakdown of the enzyme-substrate complex (ES1S2). Calculations using the HyperChem® program showed that the molecules of decanol and dodecanoic acid in the stretched form have similar sizes, equal to 1.3 ± 0.2 nm. Apparently, the carbon chains of these substrates interact with each other most effectively due to dispersion (hydrophobic) interactions between methylene (–CH2–) groups. This can lead both to specific blocking of the active site and to difficulties in the breakdown of the ES1S2 complex. As shown by numerous studies, the maximum esterification rates were observed for heptanoic acid and butanol, possibly due to the small sizes of their molecules, 0.95 and 0.6 nm, respectively. With an increase in the length of lipase substrate molecules, the efficiency of biocatalysis decreased. The broader specificity of the immobilized lipase towards the alcohol substrate indicated that the S2-binding site of the active site of the lipase was wider in size than the S1-binding site, which allowed us to confirm the conclusions about the structure of the active site of the T. lanuginosus lipase made in [27, 30–33]: the active site of the RML type lipase, to which TLL belongs, is located close to the surface of the enzyme globule and has a narrow gap for S1 (acid), and a wider one for S2 (alcohol).
In order to elucidate the mechanism of interaction between T. lanuginosus lipase and CNT, computer simulations were performed. The following objects were chosen for simulation: TLL monomer and dimer, single-walled carbon nanotubes 6.785 and 7.834 Å in diameter, with lengths of 50 and 100 Å. The following results were obtained: (1) TLL interacted with CNT through hydrophobic interactions of the following amino-acid residues: lysine, arginine, asparagine, proline, and π-cationic interactions with the lysine residue (Table 1, Fig. 5); (2) the energy of interaction between the lipase dimer and the nanotube did not depend on the CNT length and amounted to –28 kcal mol–1; (3) the energy of interaction between TLL monomer and a carbon nanotube was 2 times less than that of the dimer, since the number of bonds decreases proportionally; (4) the in silico consideration of the mobility of amino acid side residues led to a decrease in the energy by 3 kcal mol–1 (calculated per monomer); (5) CNT bound in the cleft between two lipase monomers (Fig. 6); and (6) the active sites of TLL dimer, consisting of monomers A and B, were at a distance of 6.6 and 25.8 Å (0.66 and 2.58 nm) from CNT (Fig. 6). In this case, to explain the observed maximum and minimum on the curves in Fig. 3b, it can also be assumed that both active sites of the immobilized lipase were available for low-molecular-weight C4–7 substrates, whereas for high-molecular-weight substrates with more than ten carbon atoms, one active site located at a distance of 2.58 nm from CNT was predominantly available (Fig. 6a).
Docking of the TLL dimer with a single-walled CNT 50 Å long and 7.834 Å in diameter (n = 10, m = 0). Atoms that directly interact with the CNT are indicated. Atoms 226, 227, 761, 2763, 2786, and 3295 (from Table 1) correspond to carbon atoms in CH2-group, 277 is carbon atom in CH-group.
Thus, in this work, it was found that the functional properties of the immobilized recombinant lipase rPichia/lip, such as the activity and substrate specificity, changed (modulated) depending on the chemical nature of the adsorbent. The prepared BLAs differed significantly in catalytic properties: the activity and catalytic constant for the rPichia/lip on SiO2 biocatalyst (PLSi type) were 20–30 times lower than those for rPichia/lip on MCA (PLC type). Despite the broad substrate specificity of both types of BLAs, the synthesis of ethyl esters of saturated monocarboxylic acids proceeded more efficiently with the participation of hydrophobic BLAs of the PLC type, while the synthesis of high-molecular-weight esters with pronounced hydrophobicity efficiently proceeded using the hydrophilic PLSi type. For both types of prepared biocatalysts, the maximum reaction rate was observed under esterification of heptanoic C7 acid with butyl alcohol, and the minimum, of butanoic acid with decyl C10 alcohol. The prepared active and stable biocatalysts of PLSi and PLC types undoubtedly have high practical potential for green processes of low-temperature synthesis of various esters.
REFERENCES
Mateo, C., Palomo, J.M., Fernandez-Lorente, G., Guisan, J.M., and Fernandez-Lafuente, R., Enzyme Microb. Technol., 2007, vol. 40, pp. 1451–1463.
Sokolovskii, V.D. and Kovalenko, G.A., Biotechnol. Bioeng., 1988, vol. 32, pp. 916–919.
Stoytcheva, M., Montero, G., Toscano, L., Gochev, V., and Valdez, B., in Biodiesel—Feedstocks and Processing Technologies, Stoytcheva, M. and Montero, G., Eds., InTech, 2011, pp. 397–410.
Luna, C., Garson-Perez, V., Lopez-Tenllado, F.J., Baustista, F.M., Verdugo-Escamilla, C., Aguado-Dedlas, L., et al., Catalysts, 2021, vol. 11, pp. 1350–1362. https://doi.org/10.3390/catal11111350
Hernandez-Martin, E. and Otero, C., Bioresource Technol., 2008, vol. 99, pp. 277–286.
Li, D., Adhikari, P., Shin, J.-A., Lee, J.-H., Kim, Y.-J., Zhu, X.-M., et al., Food Sci. Technol., 2010, vol. 43, pp. 458–464. https://doi.org/10.1016/j.lwt.2009.09.013
Lopez-Hernandez, A., Otero, C., Hernandez-Martin, E., Garcia, H.S., and Hill, C.G., Jr., Eur. J. Lipid Sci. Technol., 2007, vol. 109, pp. 1147–1159.
Osorio, N.M., Gusmao, J.H., Manuela da Fonseca, M., and Ferreira-Dias, S., Eur. J. Lipid Sci. Technol., 2005, vol. 107, pp. 455–463.
Handbook of Industrial Catalysis, Hou, C.T., Ed., Boca Raton, FL: CRC Press, 2005.
Bucholdz, K., Kasche, V., and Bornscheuer, U.T., Biocatalysts and Enzyme Technology, Weinheim: Wiley-VCH Verlag, 2005.
Biocatalysis for Green Chemistry and Chemical Process Development, Tao, J. and Kazlauskas, R., Eds., John Wiley and Sons, 2011.
Bezborodov, A.M., Zagustina, N.A., and Popov, V.O., Appl. Biochem. Microbiol., 2014, vol. 50, no. 4, pp. 313–337.
Cabrera, Z., Fernandez-Lorente, G., Palomo, J.M., Fernandez-Lafuente, R., and Guisan, J.M., J. Mol. Catal. B: Enzym., 2009, vol. 57, pp. 171–176.
Turati, D.F.M., Morais, W.G,Jr., Terrasan, C.R.F., Moreno-Perez, S., Pessela, B.C., Fernandez-Lorente, G., Guisan, J.M., and Carmona, E.C., Molecules, 2017, vol. 22, pp. 339–353.
Silveira, E.A., Moreno-Perez, S., Basso, A., Serban, S., Mamede, R.P., Tardioli, P.W., Farinas, C.S., et al., BMC Biotechnol., 2017, vol. 17, pp. 88–101.
Bohr, S.S., Lund, P.M., Kallenbach, F.S., Pinholt, Y., Thomsen, J., Iversen, L., et al., Sci. Rep., 2019, vol. 9, pp. 16169–16180. https://doi.org/10.1038/s41598-019-52539-1
Nurullina, P.V., Perminova, L.V., and Kovalenko, G.A., Moscow Univ. Chem. Bull., 2020, vol. 75, no. 2, pp. 110–114.
Beklemishev, A.B., Pykhtina, M.B., Perminova, L.V., and Kovalenko, G.A., Biotekhnologiya, 2021, vol. 37, no. 5, pp. 5–19. https://doi.org/10.21519/0234-2758-2021-37-5-5-62
Kovalenko, G.A., Perminova, L.V., Krasnikov, D.V., and Kuznetsov, V.L., J. Porous Mater., 2018, vol. 25, pp. 1017–1026.
Kovalenko, G.A., Perminova, L.V., and Beklemishev, A.B., React. Kinet. Mech. Catal., 2019, vol. 128, pp. 479–491. https://doi.org/10.1007/s11144-019-01648-z
Kovalenko, G.A., Perminova, L.V., Pykhina, M.B., and Beklemishev, A.B., Biocatal. Agr. Biotechnol., 2021, vol. 36, article ID 102124. https://doi.org/10.1016/j.bcab.2021.102124
Kovalenko, G.A., Perminova, L.V., Pykhina, M.B., and Beklemishev, A.B., Catal. Today, 2021, vol. 379, pp. 36–41. https://doi.org/10.1016/j.cattod.2020.11.018
Kovalenko, G.A., Perminova, L.V., Shashkov, M.V., and Beklemishev, A.B., Kinet. Catal., 2022, vol. 63, no. 2, pp. 188–196.
Bearden, J., Biochim. Biophys. Acta, 1978, vol. 533, pp. 525–529.
Kuznetsov, V.L., Suslyaev, V.I., Dorofeev, I.D., Kazakova, V.F., Moseenkov, S.I., Smirnova, T.E., and Krasnikov, D.V., Phys. Status Solidi, vol. 252, no. 11, pp. 2519–2523. https://doi.org/10.1002/pssb.201552254
RF Patent No. RU 2 577 273 C1, Byull. Izobret., 2016, no. 7.
Kumar, M., Mukherjee, J., Sinha, M., Kaur, P., Sharma, S., Gupta, M.N., and Singh, T.P., Sustain. Chem. Process., 2015, vol. 3. https://doi.org/10.1186/s40508-015-0042-5
Laane, C., Boeren, S., Vos, K., and Veeger, C., Biotechnol. Bioeng., 1987, vol. 30, pp. 81–87.
Kovalenko, G.A., Perminova, L.V., Beklemishev, A.B., Yakovleva, E.Yu., and Pykhtina, M.B., Catal. Ind., 2015, vol. 7, no. 1, pp. 73–81.
Palomo, J.M., Ortiz, C., Fuentes, M., Fernandez-Lorente, G., and Guisan, J.M., J. Chromatogr., A, 2004, vol. 1038, pp. 267–273. https://doi.org/10.1016/j.chroma.2004.03.058
Naik, S., Basu, A., Saikia, R., Madan, B., Paul, P., Chatejree, R., Brask, J., and Svendsen, A., J. Mol. Catal. B: Enzym., 2010, vol. 65, pp. 18–23. https://doi.org/10.1016/j.molcatb.2010.01.002
Fernandez-Lafuente, R., J. Mol. Catal. B: Enzym., 2010, vol. 62, pp. 197–212. https://doi.org/10.1016/j.molcatb.2009.11.010
Rodrigues, R.C. and Fernandez-Lafuente, R., J. Mol. Catal. B: Enzym., 2010, vol. 66, pp. 15–32. https://doi.org/10.1016/j.molcatb.2010.03.008
ACKNOWLEDGMENTS
The authors are grateful to Kuznetsov V.L. for the provided samples of MСA, Rudina N.A. and Ishchenko A.V. for conducting electron microscopic studies of PLC type biocatalysts.
Funding
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation within the State Task of the Boreskov Institute of Catalysis (BIC) of the Siberian Branch of the Russian Academy of Sciences (research project 0239-2021-0005), within the governmental order for BIC (project AAAA-A21-121011390007-7).
Studies on computer modeling were conducted within the State Task of the Sevastopol State University (research project FEFM-2020-0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. This article does not contain any studies involving animals performed by any of the authors.
Statement of compliance with standards of research involving humans as subjects. This article does not contain any studies involving humans as subjects.
Additional information
Translated by D. Novikova
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kovalenko, G.A., Perminova, L.V., Beklemishev, A.B. et al. Modulation of the Catalytic Properties of Immobilized Recombinant Lipase from Thermomyces lanuginosus in the Reaction of Esterification by the Selection of an Adsorbent. Appl Biochem Microbiol 58, 540–550 (2022). https://doi.org/10.1134/S000368382205009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000368382205009X