Abstract
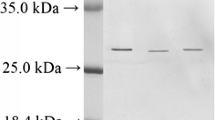
It was shown that the alkaline phosphatase (AP) isolated from eggs of the sea urchin Strongylocentrotus intermedius was a homodimer with a molecular mass of 150 kDa and that it exhibited maximal activity at a рН of 8.1–8.5 and a temperature of 45°С. Mg2+, Ca2+, and Mn2+ ions, as well as DTT, are activators of AP activity, while Zn2+, Cu2+, Cd2+, Pb2+, and Ni2+ ions, as well as EDTA, N-EMI, and p-CMB, inhibit its activity. The enzyme demonstrates unique salt resistance and can hydrolyze the substrate in seawater. It was shown by fluorescence methods and circular dichroism that an increase of the NaCl concentration above 1.0М caused noticeable changes in the secondary and tertiary structures of the protein and a decrease in enzyme activity. Analysis of the molecular masses of tryptic peptides conducted by mass spectrometry with the MASCOT program (which is based on the NCBI and SWISS-PROT databases) did not allow identification of the investigated protein. The uniqueness of the source and of the properties of the investigated AP, which were not characteristic for nonspecific AP, as well as the difficulties in primary structure identification with the fingerprinting technique, suggested that this enzyme was a nontypical AP with a novel structure.
Similar content being viewed by others
References
Coleman, J.E., Annu. Rev. Biophys. Biomol. Struct., 1992, vol. 21, no. 1, pp. 441–83.
Pfohl, R.J., Dev. Biol., 1975, vol. 44, no. 2, pp. 333–345.
Plisova, E.Yu., Balabanova, L.A., Ivanova, E.P., Kozhemyako, V.B., Mikhailov, V.V., Agafonova, E.V., and Rasskazov, V.A., Mar. Biotechnol., 2005, vol. 7, no. 3, pp. 173–178.
Zueva, N.N., Dalaev, P.G., and Lazarova, D.L., Biokhimiya, 1993, vol. 58, no. 7, pp. 1009–1023.
Asgeirsson, B., Hartemink, R., and Chlebowski, J.F., Comp. Biochem. Physiol., 1995, vol. 110 B, no. 2, pp. 315–329.
Nilsen, I.W., Overbo, K., and Olsen, R.L., Comp. Biochem. Physiol., 2001, vol. 129 B, no. 4, pp. 853–861.
Wu, H.-T., Li, D.-M., Zhu, B.-W., Cheng, J.-H., Sun, J.-J., Wang, F.-L., Yang, Y., Song, Y.-K., and Yu, C.-X., Fisheries Sci., 2013, vol. 79, no. 3, pp. 477–485.
Wende, A., Johansson, P., Ronnald, V., Dyall-Smith, M., Oesterhelt, D., and Grininger, M., J. Mol. Biol., 2010, vol. 400, no. 1, pp. 52–62.
Ishibashi, M., Yamashita, S., and Tokunaga, M., Biosci. Biotechnol. Biochem., 2005, vol. 69, no. 6, pp. 1213–1216.
Menzorova, N.I., Ivleva, A.D., Sibirtsev, Yu.T., and Rasskazov, V.A., Appl. Biochem. Microbiol., 2008, vol. 44, no. 1, pp. 106–110.
Muginova, S.V., Zhavoronkova, A.M., and Shekhovtsova, T.N., Zh. Analit. Khim., 2005, vol. 60, no. 3, pp. 247–263.
Kas’yanov, V.L. Reproduktivnaya strategiya morskikh dvustvorchatykh mollyuskov i iglokozhikh (Reproductive Strategy of Marine Bivalves and Echinoderms), Leningrad: Nauka, 1989.
Durkina, V.B. and Evtushenko, Z.S., Mar. Ecol. Progr. Ser., 1991, vol. 72, nos. 1/2, pp. 111–115.
Menzorova, N.I., Seitkalieva, A.V., and Rasskazov, V.A., Mar. Pollut. Bull., 2014, vol. 79, no. 1, pp. 188–195.
Seitkalieva, A.V., Menzorova, N.I., and Rasskazov, V.A., Russ. J. Mar. Biol., 2015, vol. 41, no. 1, pp. 51–59.
Menzorova, N.I. and Rasskazov, V.A., Biokhimiya, 1980, vol. 45, no. 3, pp. 544–553.
Bredford, M., Anal. Biochem., 1976, vol. 72, nos. 1–2, pp. 248–254.
Laemmli, U.K., Nature, 1970, vol. 227, pp. 680–685.
Chen, P.S., Toribar, N., Ir, T.Y., and Warne, H., Anal. Chem., 1956, vol. 28, no. 11, pp. 1756–1758.
Sreerama, N. and Woody, R.W., Anal. Biochem., 2000, vol. 287, no. 2, pp. 252–60.
Olsen, R.L., Overbo, K., and Myrnes, B., Comp. Biochem. Physiol., 1991, vol. 99B, no. 4, pp. 755–761.
Chuang, N.-N., Comp. Biochem. Physiol., 1990, vol. 95B, no. 1, pp. 165–169.
Homaei, A., J. Mol. Catalysis B: Enzymatic, 2015, vol. 118, pp. 16–22.
Xiao, R., Xie, L.-P., Lin, J.-Y., Li, C.-H., Chen, Q.-X., Zhou, H.-M., and Zang, R.-Q., J. Mol. Catalysis, 2002, vol. 17B, no. 2, pp. 65–74.
Liao, J.H., Chen, Q.X., Zhang, Q., Yang, Y., and Shi, Y., Appl. Biochem. Biotechnol., 2009, vol. 158, no. 2, pp. 323–333.
Hauksson, J.B., Andresson, O.S.;., and Asgeirsson, B., Enzyme Microb. Technol., 2000, vol. 27, no. 1, pp. 66–73.
Pinoni, S.A., Goldemberg, A.L., and Lopez-Mananes, A.A., J. Exp. Mar. Biol. Ecol., 2005, vol. 326, no. 2, pp. 217–226.
Lee, A.-C. and Chuang, N.-N., Comp. Biochem. Physiol., 1991, vol. 99, no. 4, pp. 845–850.
Zhang, R.-Q., Chen, Q.-X., Xiao, R., Xie, L.-P., Zeng, X.-G., and Zhou, H.-M., Biochim. Biophys. Acta, 2001, vol. 1545, no. 1, pp. 6–12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Seitkalieva, N.I. Menzorova, T.I. Vakorina, P.S. Dmitrenok, V.A. Rasskazov, 2017, published in Prikladnaya Biokhimiya i Mikrobiologiya, 2017, Vol. 53, No. 1, pp. 16–25.
Rights and permissions
About this article
Cite this article
Seitkalieva, A.V., Menzorova, N.I., Vakorina, T.I. et al. Novel salt-resistant alkaline phosphatase from eggs of the sea urchin Strongylocentrotus intermedius . Appl Biochem Microbiol 53, 11–19 (2017). https://doi.org/10.1134/S0003683817010173
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683817010173