Abstract
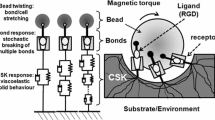
We attempted to estimate in living adherent epithelial alveolar cells, the degree of structural and mechanical heterogeneity by considering two individualized cytoskeleton components, i.e., a submembranous “cortical” cytoskeleton and a “deep” cytoskeleton (CSK). F-actin structure characterizing each CSK component was visualized from spatial reconstructions at low and high density, respectively, especially in a 10-μm-cubic neighborhood including the bead. Specific mechanical properties (Young elastic and viscous modulus E and η) were revealed after partitioning the magnetic twisting cytometry response using a double viscoelastic “solid” model with asymmetric plastic relaxation. Results show that the cortical CSK response is a faster (τ 1≤ 0.7s), softer (E1: 63-109 Pa), moderately viscous (η1: 7-18 Pa s), slightly tensed, and easily damaged structure compared to the deep CSK structure which appears slower (τ2 ∼ \( \frac{1}{2} \) min), stiffer (E2: 95-204 Pa), highly viscous (η2: 760-1967 Pa s), more tensed, and fully elastic, while exhibiting a larger stress hardening behavior. Adding drug depolymerizing actin filaments decreased predominantly the deep CSK stiffness. By contrast, an agent altering cell–matrix interactions affected essentially the cortical CSK stiffness. We concluded that partitioning the CSK within cortical and deep structures is largely consistent with their respective functional activities. © 2003 Biomedical Engineering Society.
PAC2003: 8716Ka, 8716Ac, 8380Lz
Similar content being viewed by others
References
Albrecht-Buehler, G. Role of cortical tension in fibroblast shape and movement. Cell Motil. Cytoskeleton7:54–67, 1987.
Bausch, A. R., U. Hellerer, M. Essler, M. Aepfelbacher, and E. Sackmann. Rapid stiffening of integrin receptor–actin linkages in endothelial cells stimulated with thrombin: A magnetic bead microrheology study. Biophys. J. 80:2649–2657, 2001.
Bausch, A. R., W. Möller, and E. Sackmann. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys. J. 76:573–579, 1999.
Bausch, A. R., F. Ziemann, A. A. Boulbitch, K. Jacobson, and E. Sackmann. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophys. J. 75:2038–2049, 1998.
Cañadas, P., V. M. Laurent, C. Oddou, D. Isabey, and S. Wendling. A cellular tensegrity model to analyze the structural viscoelasticity of the cytoskeleton. J. Theor. Biol. 218:155–173, 2002.
Cheng, Y., C. A. Hartemink, J. H. Hartwig, and C. F. Dewey, Jr. Three-dimensional reconstruction of the actin cytoskeleton from stereo images. J. Biomech. 33:105–113, 2000.
Chicurel, M. E., C. S. Chen, and D. E. Ingber. Cellular control lies in the balance of forces. Curr. Opin. Cell Biol. 10:232–239, 1998.
Choquet, D., D. P. Felsenfeld, and M. P. Sheetz. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell88:39–48, 1997.
DePina, A. S., and G. M. Langford. Vesicle transport: the role of actin filaments and myosin motors. Microsc. Res. Tech. 47:93–106, 1999.
Dong, C., R. Skalak, K. L. Sung, G. W. Schmid-Schonbein, and S. Chien. Passive deformation analysis of human leukocytes. J. Biomech. Eng. 110:27–36, 1988.
Elson, E. L. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu. Rev. Biophys. Biophys. Chem. 17:397–430, 1988.
Evans, E., and A. Yeung. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys. J. 56:151–160, 1989.
Fabry, B., G. Maksym, R. Hubmayr, J. Butler, and J. Fredberg. Implications of heterogeneous bead behavior on cell mechanical properties measured with magnetic twisting cytometry. J. Magn. Magn. Mater. 194:120–125, 1999.
Fabry, B., G. N. Maksym, J. P. Butler, M. Glogauer, D. Navajas, and J. J. Fredberg. Scaling the microrheology of living cells. Phys. Rev. Lett. 87:148102, 2001.
Forgacs, G. On the possible role of cytoskeletal filamentous networks in intracellular signaling: An approach based on percolation. J. Cell. Sci. 108:2131–2143, 1995.
Glogauer, M., P. Arora, G. Yao, I. Sokholov, J. Ferrier, and C. A. McCulloch. Calcium ions and tyrosine phosphorylation interact coordinately with actin to regulate cytoprotective responses to stretching. J. Cell. Sci. 110:11–21, 1997.
Goode, B. L., D. G. Drubin, and G. Barnes. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 12:63–71, 2000.
Hamill, O. P., and B. Martinac. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740, 2001.
Hartwig, J. H., and P. Shevlin. The architecture of actin filaments and the ultrastructural location of actin-binding protein in the periphery of lung macrophages. J. Cell Biol. 103:1007–1020, 1986.
Heidemann, S. R., S. Kaech, R. E. Buxbaum, and A. Matus. Direct observations of the mechanical behaviors of the cytoskeleton in living fibroblasts. J. Cell Biol. 145:109–122, 1999.
Hochmuth, R. M., P. R. Worthy, and E. A. Evans. Red cell extensional recovery and the determination of membrane viscosity. Biophys. J. 26:101–114, 1979.
Holley, M. C., and J. F. Ashmore. A cytoskeletal spring in cochlear outer hair cells. Nature (London)335:635–637, 1988.
Ingber, D. E. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J. Cell. Sci. 104:613–627, 1993.
Ingber, D. E. Opposing views on tensegrity as a structural framework for understanding cell mechanics. J. Appl. Physiol. 89:1663–1670, 2000.
Ingber, D. E., L. Dike, L. Hansen, S. Karp, H. Liley, A. Maniotis, H. McNamee, D. Mooney, G. Plopper, and J. Sims. Cellular tensegrity: Exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int. Rev. Cytol. 150:173–224, 1994.
Ingber, D. E., D. Prusty, Z. Sun, H. Betensky, and N. Wang. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J. Biomech. 28:1471–1484, 1995.
Janmey, P. A. The cytoskeleton and cell signaling: Component localization and mechanical coupling. Physiol. Rev. 78:763–781, 1998.
Katoh, K., Y. Kano, M. Masuda, H. Onishi, and K. Fujiwara. Isolation and contraction of the stress fiber. Mol. Biol. Cell9:1919–1938, 1998.
Katoh, K., M. Masuda, Y. Kano, Y. Jinguji, and K. Fujiwara. Focal adhesion proteins associated with apical stress fibers of human fibroblasts. Cell Motil. Cytoskeleton31:177–195, 1995.
Kaverina, I., O. Krylyshkina, and J. V. Small. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146:1033–1044, 1999.
Knauper, V., C. Lopez-Otin, B. Smith, G. Knight, and G. Murphy. Biochemical characterization of human collagenase-3. J. Biol. Chem. 271:1544–1550, 1996.
Koffer, A., P. E. Tatham, and B. D. Gomperts. Changes in the state of actin during the exocytotic reaction of permeabilized rat mast cells. J. Cell Biol. 111:919–927, 1990.
Laurent, V. M., S. Henon, E. Planus, R. Fodil, M. Balland, D. Isabey, and F. Gallet. Assessment of mechanical properties of adherent living cells by bead micromanipulation: Comparison of magnetic twisting cytometry versus optical tweezers. J. Biomech. Eng. 124:408–421, 2002.
Luby-Phelps, K. Physical properties of cytoplasm. Curr. Opin. Cell Biol. 6:3–9, 1994.
Maksym, G. N., B. Fabry, J. P. Butler, D. Navajas, D. J. Tschumperlin, J. D. Laporte, and J. J. Fredberg. Mechanical properties of cultured human airway smooth muscle cells from 0.05 to 0.4 Hz. J. Appl. Physiol. 89:1619–1632, 2000.
Maniotis, A. J., C. S. Chen, and D. E. Ingber. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. U.S.A. 94:849–854, 1997.
Mathur, A. B., G. A. Truskey, and W. M. Reichert. Atomic force and total reflection fluorescence microscopy for the study of force transmission in endothelial cells. Biophys. J. 78:1725–1735, 2000.
Mijailovich, S. M., M. Kojic, M. Zivkovic, B. Fabry, and J. J. Fredberg. A finite element model of cell deformation during magnetic bead twisting. J. Appl. Physiol. 93:1429–1436, 2002.
Muallem, S., K. Kwiatkowska, X. Xu, and H. L. Yin. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol. 128:589–598, 1995.
Murphy, G., J. A. Allan, F. Willenbrock, M. I. Cockett, J. P. O'Connell, and A. J. Docherty. The role of the C-terminal domain in collagenase and stromelysin specificity. J. Biol. Chem. 267:9612–9618, 1992.
Planus, E., S. Galiacy, M. Matthay, V. Laurent, J. Gavrilovic, G. Murphy, C. Clérici, D. Isabey, C. Lafuma, and M. P. d'Ortho. Role of collagenase in mediating alveolar epithelial wound repair. J. Cell. Sci. 112(2):243–252, 1999.
Potard, U. S., J. P. Butler, and N. Wang. Cytoskeletal mechanics in confluent epithelial cells probed through integrins and E-cadherins. Am. J. Physiol. 272:C1654–C1663, 1997.
Pourati, J., A. Maniotis, D. Spiegel, J. L. Schaffer, J. P. Butler, J. J. Fredberg, D. E. Ingber, D. Stamenovic, and N. Wang. Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells?Am. J. Physiol. 274:C1283–C1289, 1998.
Qualmann, B., M. M. Kessels, and R. B. Kelly. Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 150:F111–F116, 2000.
Raucher, D., T. Stauffer, W. Chen, K. Shen, S. Guo, J. D. York, M. P. Sheetz, and T. Meyer. Phosphatidylinositol 4,5–bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell100:221–228, 2000.
Satcher, R. L. J., and C. F. J. Dewey. Theoretical estimates of mechanical properties of the endothelial cell cytoskeleton (see comments). Biophys. J. 71:109–118, 1996.
Sato, M., D. P. Theret, L. T. Wheeler, N. Ohshima, and R. M. Nerem. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties. J. Biomech. Eng. 112:263–268, 1990.
Small, J. V. The actin cytoskeleton. Electron Microsc. Rev. 1:155–174, 1988.
Small, J. V., K. Rottner, I. Kaverina, and K. I. Anderson. Assembling an actin cytoskeleton for cell attachment and movement. Biochim. Biophys. Acta1404:271–281, 1998.
Stamenovic, D., D. E. Ingber, N. Wang, and J. J. Fredberg. A microstructural approach to cytoskeletal mechanics based on tensegrity. J. Theor. Biol. 181:125–136, 1996.
Sung, K. L., C. Dong, G. W. Schmid-Schonbein, S. Chien, and R. Skalak. Leukocyte relaxation properties. Biophys. J. 54:331–336, 1988.
Wang, N. Mechanical interactions among cytoskeletal filaments. Hypertension32:162–165, 1998.
Wang, N., J. P. Butler, and D. E. Ingber. Mechanotransduction across the cell surface and through the cytoskeleton. Science260:1124–1127, 1993.
Wang, N., and D. E. Ingber. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys. J. 66:2181–2189, 1994.
Wendling, S., C. Oddou, and D. Isabey. Stiffening response of a cellular tensegrity model. J. Theor. Biol. 196:309–325, 1999.
Wendling, S., E. Planus, V. Laurent, L. Barbe, A. Mary, C. Oddou, and D. Isabey. Role of cellular tone and microenvironment on cytoskeleton stiffness predicted by tensegrity model. Eur. Phys. J.: Appl. Phys. 9:51–62, 2000.
Wu, Z., K. Wong, M. Glogauer, R. P. Ellen, and C. A. McCulloch. Regulation of stretch-activated intracellular calcium transients by actin filaments. Biochem. Biophys. Res. Commun. 261:419–425, 1999.
Yamada, S., D. Wirtz, and S. C. Kuo. Mechanics of living cells measured by laser tracking microrheology. Biophys. J. 78:1736–1747, 2000.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laurent, V.M., Fodil, R., Cañadas, P. et al. Partitioning of Cortical and Deep Cytoskeleton Responses from Transient Magnetic Bead Twisting. Annals of Biomedical Engineering 31, 1263–1278 (2003). https://doi.org/10.1114/1.1616932
Issue Date:
DOI: https://doi.org/10.1114/1.1616932