Abstract
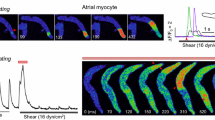
Vascular smooth muscle cells (VSM) are normally exposed to transmural fluid flow shear stresses, and after vascular injury, blood flow shear stresses are imposed upon them. Since Ca2+ is a ubiquitous intracellular signaling molecule, we examined the effects of fluid flow on intracellular Ca2+ concentration in rat aortic smooth muscle cells to assess VSM responsiveness to shear stress. Cells loaded with fura 2 were exposed to steady flow shear stress levels of 0.5–10.0 dyn/cm2 in a parallel-plate flow chamber. The percentage of cells displaying a rise in cytosolic Ca2+ ion concentration ([Ca2+]i) increased in response to increasing flow, but there was no effect of flow on the ([Ca2+]i) amplitude of responding cells. Addition of Gd3+ (10 μM) or thapsigargin (50 nM) significantly reduced the percentage of cells responding and the response amplitude, suggesting that influx of Ca2+ through ion channels and release from intracellular stores contribute to the rise in ([Ca2+]i) in response to flow. The addition of nifedipine (1 or 10 μM) or ryanodine (10 μM) also significantly reduced the response amplitude, further defining the role of ion channels and intracellular stores in the Ca2+ response. © 2002 Biomedical Engineering Society.
PAC2002: 8716Uv, 8719Uv, 8716Dg, 8719Ff
Similar content being viewed by others
REFERENCES
Alshihabi, S. N., Y. S. Chang, J. A. Frangos, and J. M. Tarbel. Shear stress–induced release of PGE2 and PGI2 by vascular smooth muscle. Biochem. Biophys. Res. Commun. 224:808–824, 1996.
Bean, B. P. Classes of calcium channels in vertebrate cells. Annu. Rev. Physiol. 51:367–384, 1989.
Biagi, B. A., and J. J. Enyeart. Gadolinium blocks low–and high–threshold calcium currents in pituitary cells. Am. J. Physiol. 259:C515–C520, 1990.
Buck, R. C. Behavior of vascular smooth muscle cells during repeated stretching of the substratum in vivo. Atherosclerosis 46:217–223, 1983.
Clowes, A. W., T. R. Kirkman, and M. M. Clowes. Mechanisms of arterial graft failure 4. Chronic endothelial and smooth muscle cell proliferation in healing polytetrafluoroethylene protheses. J. Vasc. Surg. 3:877–884, 1986.
Davies, P.. Flow–mediated endothelial mechanotranduction. Phys. Rev. 75:519–560, 1995.
Davis, M. J., and L. M. Burch. Single stretch–activated ion channels in vascular smooth muscle cells. FASEB J. 3:A254, 1989.
Davis, M. J., G. A. Meininger, and D. C. Zawieja. Stretch–induced increases in intracellular calcium of isolated vascular smooth muscle cells. Am. J. Physiol. 263:H1292–H1299, 1992.
Downing, S. D., and D. F. Socie. Simple rainflow counting algorithms. Int. J. Fatigue 4:31–40, 1982.
Dull, R., and P. F. Davies. Flow modulation of agonist (ATP)–response [Ca2+] coupling in vascular endothelium. Am. J. Physiol. 261:H149–H154, 1991.
Dull, R., J. M. Tarbell, and P. Davies. Mechanisms of flow–mediated signal transduction in endothelial cells: Kinetics of ATP surface concentrations. J. Vasc. Res. 29:410–419, 1992.
Frangos, J. A., L. V. McIntire, and S. G. Eskin. Shear stress–induced stimulation of mammalian cell metabolism. Biotechnol. Bioeng. 32:1053–1060, 1988.
Ganitkevich, V. Y., and G. Isenberg. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea pig coronary artery. J. Physiol. (London) 426:19–42, 1990.
Geary, R. L., T. R. Kohler, S. Vergel, T. R. Kirkman, and A. W. Clowes. Time course of flow–induced smooth muscle cell proliferation and intimal thickening in endothelialized baboon vascular grafts. Circ. Res. 74:14–23, 1993.
Geiger, R. V., B. C. Berk, R. W. Alexander, and R. M. Nerem. Flow–induced calcium transients in single endothelial cells: Spatial and temporal analysis. Am. J. Physiol. 262:C1411–C1417, 1992.
Grynkiewicz, G., M. Poenie, and R. Tsien. A new generation of Ca2+ indicators with greatly improved fluorescene properties. J. Biol. Chem. 260:3440–3450, 1985.
Helminger, G. B., C. Berk, and R. M. Nerem. Calcium responses of endothelial cell monolayers subjected to pulsatile and steady laminar flow differ. Am. J. Physiol. 269:C367–C375, 1995.
Hisayama, T., I. Takayanagi, and Y. Okamoto. Ryanodine reveals multiple contractile and relaxant mechanisms in vascular smooth muscle: Simultaneous measurements of mechanical activity and cytoplasmic free Ca2+ level with fura–2. Br. J. Pharmacol. 100:677–684, 1990.
Hung, C. T., F. D. Allen, S. R. Pollack, and C. T. Brighton. Intracellular Ca2+ stores and extracellular Ca2+ stores are required in the real–time Ca2+ response of bone cells experiencing fluid flow. J. Biomech. 29:1411–1417, 1996.
Hung, C. T., S. R. Pollack, M. Reilly, and C. T. Brighton. Real–time calcium response of cultured bone cells to fluid flow. Clin. Orthop. Relat. Res. 313:256–269, 1995.
Iino, M., T. Kobayaski, and M. Endo. Use of ryanodine for functional removal of the calcium store in smooth muscle cells of the guinea pig. Biochem. Biophys. Res. Commun. 254:417–422, 1988.
Jacobs, C. R., C. E. Yellowley, B. R. Davis, Z. Zhou, J. M. Cimbala, and H. J. Donahue. Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 11:969–976, 1998.
Kirber, M. T., L. H. Clapp, A. M. Gurney, J. V. Walsh, Jr., and J. J. Singer. Stretch–activated ion channels in mammalian vascular smooth muscle cells. J. Gen. Physiol. 94:37a–38a, 1989.
Kirber, M. T., J. V. Walsh, Jr., and J. J. Singer. Stretch–activated ion channels in smooth muscle: A mechanism for the initiation of stretch–induced contraction. Pfluegers Arch. 412:339–345, 1988.
Kulik, T. J., R. A. Bialecki, W. S. Colucci, A. Rothman, E. T. Glennon, and R. H. Underwood. Stretch increases inositol triphosphate and inositol tetrakiphosphate in cultured pulmonary vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 180:982–987, 1991.
Lacampagne, A., F. Gannier, J. Argibay, D. Garnier, and J. Y. Guennec. The stretch–activated ion channel blocker gadolinium also blocks L–type calcium channels in isolated ventricular myocytes of the guinea pig. Biochim. Biophys. Acta 1191:205–208, 1994.
Laine, M., O. Arjamaa, O. Vuolteenaho, H. Ruskoaho, and M. Weckstrom. Block of stretch–activated atrial natriuretic peptide secretion by gadolinium in isolated rat atrium. J. Physiol. (Paris) 480:353–361, 1994.
Li, C., and Q. Xu. Mechanical stress–initiated signal transduction in vascular smooth muscle cells. Cell. Signal. 12:435–445, 2000.
Leung, D. Y., S. Glagov, and M. B. Mathews. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191:475–477, 1976.
Lever, M. J., J. M. Tarbell, and C. G. Caro. The effect of luminal flow in rabbit carotid artery on transmural fluid transport. Exp. Physiol. 77:553–563, 1992.
Mishra, S., and K. Hermsmeyer. Selective inhibition of T–type Ca2+ channels by RO 40–5967. Circ. Res. 75:144–148, 1994.
Papadaki, M., M. G. Tilton, S. J. Eskin, and L. V. McIntire. Nitric oxide production by cultured human aortic smooth muscle cells: Stimulation by fluid flow. Am. J. Physiol. 274:H616–H626, 1998.
Potocnik, S. J., T. V. Murphy, N. Kotecha, and M. Hill. Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca2+. Br. J. Pharmacol. 131:1065–1072, 2000.
Setoguchi, M., Y. Ohya, I. Abe, and M. Fujishima. Stretch–activated whole–cell currents in smooth muscle cells from mesenteric resistance artery of guinea pig. J. Physiol. (Paris) 501:343–353, 1997.
Sill, H. W., Y. S. Chang, J. R. Artman, J. A. Frangos, T. M. Hollis, and J. M. Tarbell. Shear stress increases hydraulic conductivity of cultured endothelial monolayers. Am. J. Physiol. 268:H535–H543, 1995.
Sterpetti, A., A. Cucina, L. S. D'Angelo, B. Cardillo, and A. Cavallaro. Response of arterial smooth muscle cells to laminar flow. J. Cardiovasc. Surg. 33:619–624, 1992.
Tada, S., and J. M. Tarbell. Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am. J. Physiol. 278:H1489–H1497, 2000.
Wagner, C. T., W. Durante, N. Christodoulides, J. D. Hellums, and A. I. Schafer. Hemodynamic forces induce the expression of heme oxygenase in cultured vascular smooth muscle cells. J. Clin. Invest. 100:589–596, 1997.
Wang, D. M., and J. M. Tarbell. Modeling interstitial flow in an artery wall allow estimation of wall shear stress on smooth muscle cells. J. Biomech. Eng. 117:358–363, 1995.
Wang, S., and J. M. Tarbell. Effects of fluid flow on smooth muscle cells in a three–dimensional collagen gel model. Arterioscler., Thromb., Vasc. Biol. 20:2220–2225, 2000.
Wilkinson, N. C., F. Gao, and O. P. Hamill. Effects of mechanogated cation channel blockers on xenopus oocyte growth and development. J. Membr. Biol. 165:161–174, 1998.
Wingertzahn, M. A., and S. O. Raymond. Changes in ryanodine receptor–mediated calcium release during skeletal muscle differentiation. Exp. Biol. Med. 226:119–126, 2001.
Xuan, Y. T., O. L. Wang, and A. R. Whorton. Thapsigargin stimulates Ca2+ entry in vascular smooth muscle cells: Nicardipine–sensitive and–insensitive pathways. Am. J. Physiol. 262:C1258–C1265, 1992.
Yellowley, C. E., C. R. Jacobs, Z. Li, Z. Zhou, and H. J. Donahue. Effects of fluid flow on intracellular calcium in bovine articular chondrocytes. Am. J. Physiol. 273:C30–C36, 1997.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sharma, R., Yellowley, C.E., Civelek, M. et al. Intracellular Calcium Changes in Rat Aortic Smooth Muscle Cells in Response to Fluid Flow. Annals of Biomedical Engineering 30, 371–378 (2002). https://doi.org/10.1114/1.1470179
Issue Date:
DOI: https://doi.org/10.1114/1.1470179