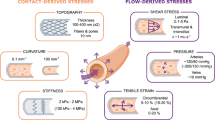
Abstract
The endothelium of blood vessels presents a wavy surface to the flowing blood. The subcellular distribution of shear stress depends on the shape and orientation of the cells and on their spatial arrangement within the monolayer. By studying details of the distribution of stress at this scale and the morphological responses that serve to modify the distribution, we can gain insight into the physical mechanisms by which the cell senses its fluid mechanical environment. The rapidly growing body of evidence indicates that endothelial cells discriminate between subtle variations in the exact loading conditions including differences in temporal and spatial gradients of shear stress, steady and pulsatile laminar flow, and laminar and turbulent flows. While in a few studies the effects of these individual flow characteristics have been carefully isolated, it is difficult to assess the relative importance of any one parameter. To interpret the relationships between isolated flow characteristics or the integrated effects of combined loading conditions and the biochemical signaling events that mediate the cell response, a full stress analysis of the cell is needed. The microscopic distribution of shear stress acting upon the cell surface provides the boundary condition for such an analysis. Experimental and analytical tools are being developed to assess the stress distribution throughout the cellular structures that might be involved in mechanotransduction. © 2002 Biomedical Engineering Society.
PAC2002: 8716Xa, 8719Uv, 8719Xx
Similar content being viewed by others
REFERENCES
Bao, X., C. B. Clark, and J. A. Frangos. Temporal gradient in shear-induced signaling pathway: Involvement of MAP kinase, c-fos, and connexin43. Am. J. Physiol. 278:H1598–H1605, 2000.
Bao, X., C. Lu, and J. A. Frangos. Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: Role of NO, NF kappa B, and egr-1. Arterioscler., Thromb., Vasc. Biol. 19:996–1003, 1999.
Barbee, K. A. Changes in surface topography in endothelial monolayers with time at confluence: Influence on subcellular shear stress distribution due to flow. Biochem. Cell Biol. 73:501–505, 1995.
Barbee, K. A., P. F. Davies, and R. Lal. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ. Res. 74:163–171, 1994.
Barbee, K. A., T. Mundel, R. Lal, and P. F. Davies. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am. J. Physiol. 268:H1765–H1772, 1995.
Berk, B. C., M. A. Corson, T. E. Peterson, and H. Tseng. Protein kinases as mediators of fluid shear stress stimulated signal transduction in endothelial cells: A hypothesis for calcium-dependent and calcium-independent events activated by flow. J. Biomech. 28:1439–1450, 1995.
Binnig, G., C. F. Quate, and C. Gerber. Atomic force microscope. Phys. Rev. Lett. 56:930–933, 1986.
Blackman, B. R., S. Aravind, T. N. Tulenko, and K. A. Barbee. Cholesterol suppresses endothelial cell calcium response to shear stress. Ann. Biomed. Eng. 26:S27, 2001.
Blackman, B. R., K. A. Barbee, and L. E. Thibault. In vitro cell shearing device to investigate the dynamic response of cells in a controlled hydrodynamic environment. Ann. Biomed. Eng. 28:363–372, 2000.
Blackman, B. R., L. E. Thibault, and K. A. Barbee. Selective modulation of endothelial cell [Ca2+]iresponse to flow by the onset rate of shear stress. J. Biomech. Eng. 122:274–282, 2000.
Butler, P. J., G. Norwich, S. Weinbaum, and S. Chien. Shear stress induces a time-and position-dependent increase in endothelial cell membrane fluidity. Am. J. Physiol. 280:C962–C969, 2001.
Butler, P. J., S. Weinbaum, S. Chien, and D. E. Lemons. Endothelium-dependent, shear-induced vasodilation is ratesensitive. Microcirculation (Philadelphia) 7:53–65, 2000.
Chien, S., M. M. Lee, L. S. Laufer, D. A. Handley, S. Weinbaum, C. G. Caro, and S. Usami. Effects of oscillatory mechanical disturbance on macromolecular uptake by arterial wall. Arteriosclerosis (Dallas) 1:326–336, 1981.
Davies, P. F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75:519–560, 1995.
Davies, P. F., C. F. Dewey, Jr., S. R. Bussolari, E. J. Gordon, and M. A. Gimbrone, Jr. Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J. Clin. Invest. 73:1121–1129, 1984.
Davies, P. F., T. Mundel, and K. A. Barbee. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J. Biomech. 28:1553–1560, 1995.
Davies, P. F., D. C. Polacek, J. S. Handen, B. P. Helmke, and N. DePaola. A spatial approach to transcriptional profiling: Mechanotransduction and the focal origin of atherosclerosis. Trends Biotechnol. 17:347–351, 1999.
Davies, P. F., A. Remuzzi, E. J. Gordon, C. F. Dewey, Jr., and M. A. Gimbrone, Jr. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc. Natl. Acad. Sci. U.S.A. 83:2114–2117, 1986.
Davies, P. F., A. Robotewskyj, and M. L. Griem. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J. Clin. Invest. 93:2031–2038, 1994.
DePaola, N., P. F. Davies, W. F. Pritchard, Jr., L. Florez, N. Harbeck, and D. C. Polacek. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc. Natl. Acad. Sci. U.S.A. 96:3154–3159, 1999.
DePaola, N., M. A. Gimbrone, Jr., P. F. Davies, and C. F. Dewey, Jr. Vascular endothelium responds to fluid shear stress gradients. Arterioscler. Thromb. 12:1254–1257, 1992.
Eberwine, J., H. Yeh, K. Miyashiro, Y. Cao, S. Nair, R. Finnell, M. Zettel, and P. Coleman. Analysis of gene expression in single live neurons. Proc. Natl. Acad. Sci. U.S.A. 89:3010–3014, 1992.
Flaherty, J. T., J. E. Pierce, V. J. Ferrans, D. J. Patel, W. K. Tucker, and D. L. Fry. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ. Res. 30:23–33, 1972.
Frangos, J. A., T. Y. Huang, and C. B. Clark. Steady shear and step changes in shear stimulate endothelium via independent mechanisms—Superposition of transient and sustained nitric oxide production. Biochem. Biophys. Res. Commun. 224:660–665, 1996.
Fukushima, S., T. Deguchi, M. Kaibara, K. Oka, and K. Tanishita, Microscopic velocimetry with a scaled-up model for evaluating a flow field over cultured endothelial cells. J. Biomech. Eng. (in press).
Fung, Y. C. Foundations of Solid Mechanics. Englewood Cliffs, NJ: Prentice-Hall, 1965.
Fung, Y. C., and S. Q. Liu. Elementary mechanics of the endothelium of blood vessels. J. Biomech. Eng. 115:1–12, 1993.
Garcia-Cardena, G., J. Comander, K. R. Anderson, B. R. Blackman, and M. A. Gimbrone, Jr. Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc. Natl. Acad. Sci. U.S.A. 98:4478–4485, 2001.
Glagov, S., C. K. Zarins, N. Masawa, C. P. Xu, H. Bassiouny, and D. P. Giddens. Mechanical functional role of nonatherosclerotic intimal thickening. Front Med. Biol. Eng. 5:37–43, 1993.
Gudi, S., J. P. Nolan, and J. A. Frangos. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc. Natl. Acad. Sci. U.S.A. 95:2515–2519, 1998.
Haidekker, M. A., N. L'Heureux, and J. A. Frangos. Fluid shear stress increases membrane fluidity in endothelial cells: A study with DCVJ fluorescence. Am. J. Physiol. 278:H1401–H1406, 2000.
Haidekker, M. A., T. Ling, M. Anglo, H. Y. Stevens, J. A. Frangos, and E. A. Theodorakis. New fluorescent probes for the measurement of cell membrane viscosity. Chem. Biol. 8:123–131, 2001.
Hassan, E., W. F. Heinz, M. D. Antonik, N. P. D'Costa, S. Nageswaran, C. A. Schoenenberger, and J. H. Hoh. Relative microelastic mapping of living cells by atomic force microscopy. Biophys. J. 74:1564–1578, 1998.
Helmke, B. P., R. D. Goldman, and P. F. Davies. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ. Res. 86:745–752, 2000.
Helmke, B. P., D. B. Thakker, R. D. Goldman, and P. F. Davies. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys. J. 80:184–194, 2001.
Helmlinger, G., R. V. Geiger, S. Schreck, and R. M. Nerem. Effects of pulsatile flow on cultured vascular endothelial cell morphology. J. Biomech. Eng. 113:123–131, 1991.
Holland, J. A., K. A. Pritchard, N. J. Rogers, and M. B. Stemerman. Perturbation of cultured human endothelial cells by atherogenic levels of low density lipoprotein. Am. J. Pathol. 132:474–478, 1988.
James, S. R., A. Paterson, T. K. Harden, R. A. Demel, and C. P. Downes. Dependence of the activity of phospholipase C beta on surface pressure and surface composition in phospholipid monolayers and its implications for their regulation. Biochemistry 36:848–855, 1997.
Kamiya, A., and T. Togawa. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. 239:H14–H21, 1980.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis (Dallas) 5:293–302, 1985.
Kuchan, M. J., and J. A. Frangos. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 266:C628–C636, 1994.
Lal, R., and S. A. John. Biological applications of atomic force microscopy. Am. J. Physiol. 266:C1–21, 1994.
Langille, B. L., and F. O'Donnell. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231:405–407, 1986.
Laury-Kleintop, L. D., M. Gleason, and T. N. Tulenko. Expression of the heterogenous nuclear ribonucleoprotein complex K protein and the prolyl-4-hydroxylase alpha-subunit in atherosclerotic arterial smooth muscle cells. Biochem. Biophys. Res. Commun. 260:382–389, 1999.
Liu, S. Q., M. Yen, and Y. C. Fung. On measuring the third dimension of cultured endothelial cells in shear flow. Proc. Natl. Acad. Sci. U.S.A. 91:8782–8786, 1994.
Lundbaek, J. A., P. Birn, J. Girshman, A. J. Hansen, and O. S. Andersen. Membrane stiffness and channel function. Biochemistry 35:3825–3830, 1996.
Malinauskas, R. A., P. Sarraf, K. M. Barber, and G. A. Truskey. Association between secondary flow in models of the aorto-celiac junction and subendothelial macrophages in the normal rabbit. Atherosclerosis 140:121–134, 1998.
Mathur, A. B., G. A. Truskey, and W. M. Reichert. Total internal reflection microscopy and atomic force microscopy (TIRFM-AFM) to study stress transduction mechanisms in endothelial cells. Crit. Rev. Biomed. Eng. 28:197–202, 2000.
Meinhart, C. D., S. T. Werely, and J. G. Santiago. PIV measurements of a microchannel flow. Exp. Fluids 27:414–419, 1999.
Morita, T., H. Kurihara, K. Maemura, M. Yoshizumi, and Y. Yazaki. Disruption of cytoskeletal structures mediates shear stress-induced endothelin-1 gene expression in cultured porcine aortic endothelial cells. J. Clin. Invest. 92:1706–1712, 1993.
Nerem, R. M., M. J. Levesque, and J. F. Cornhill. Vascular endothelial morphology as an indicator of the pattern of blood flow. J. Biomech. Eng. 103:172–176, 1981.
Ojha, M. Spatial and temporal variations of wall shear stress within an end-to-side arterial anastomosis model. J. Biomech. 26:1377–1388, 1993.
Plopper, G., and D. E. Ingber. Rapid induction and isolation of focal adhesion complexes. Biochem. Biophys. Res. Commun. 193:571–578, 1993.
Pohl, U., J. Holtz, R. Busse, and E. Bassenge. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8:37–44, 1986.
Rebecchi, M., M. Bonhomme, and S. Scarlata. Role of lipid packing in the activity of phospholipase C-δ 1 as determined by hydrostatic pressure measurements. Biochem. J. 341:571–576, 1999.
Remuzzi, A., C. F. Dewey, Jr., P. F. Davies, and M. A. Gimbrone, Jr.. Orientation of endothelial cells in shear fields in vitro. Biorheology 21:617–630, 1984.
Rizzo, V., D. P. McIntosh, P. Oh, and J. E. Schnitzer. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J. Biol. Chem. 273:34724–34729, 1998.
Satcher, Jr., R. L., S. R. Bussolari, M. A. Gimbrone, Jr., and C. F. Dewey, Jr. The distribution of fluid forces on model arterial endothelium using computational fluid dynamics. J. Biomed. Eng. 114:309–316, 1992.
Sato, M., K. Nagayama, N. Kataoka, M. Sasaki, and K. Hane. Local mechanical properties measured by atomic force microscopy for cultured bovine endothelial cells exposed to shear stress. J. Biomech. 33:127–135, 2000.
Smalley, D. M., J. H. Lin, M. L. Curtis, Y. Kobari, M. B. Stemerman, and K. A. Pritchard, Jr. Native LDL increases endothelial cell adhesiveness by inducing intercellular adhesion molecule-1. Arterioscler., Thromb., Vasc. Biol. 16:585–590, 1996.
Walpola, P. L., A. I. Gotlieb, M. I. Cybulsky, and B. L. Langille. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler., Thromb., Vasc. Biol. 15:2–10, 1995.
Wang, N., J. P. Butler, and D. E. Ingber. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260:1124–1127, 1993.
Yamada, S., D. Wirtz, and S. C. Kuo. Mechanics of living cells measured by laser tracking microrheology. Biophys. J. 78:1736–1747, 2000.
Yamaguchi, T., K. Hoshiai, H. Okino, A. Sakurai, S. Hanai, M. Masuda, and Fujiwara. Shear stress distribution over confluently cultured endothelial cells studied by computational fluid mechanics. ASME Adv. Bioeng. BED 29:167–170,1993.
Yamaguchi, T., Y. Yamamoto, and H. Liu. Computational mechanical model studies on the spontaneous emergent morphogenesis of the cultured endothelial cells. J. Biomech. 33:115–126, 2000.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barbee, K.A. Role of Subcellular Shear–Stress Distributions in Endothelial Cell Mechanotransduction. Annals of Biomedical Engineering 30, 472–482 (2002). https://doi.org/10.1114/1.1467678
Issue Date:
DOI: https://doi.org/10.1114/1.1467678