Abstract
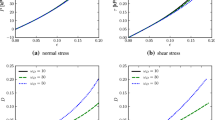
Very large amplitude pseudorandom uniaxial perturbations containing frequencies between 0.125 and 12.5 Hz were applied to five dog lung tissue strips. Three different nonlinear block-structured models in nonparametric form were fit to the data. These models consisted of (1) a static nonlinear block followed by a dynamic linear block (Hammerstein model); (2) the same blocks in reverse order (Wiener model); and (3) the blocks in parallel (parallel model). Both the Hammerstein and Wiener models performed well for a given input perturbation, each accounting for greater than 99% of the measured stress signal variance. However, the Wiener and parallel model parameters showed some dependence on the strain amplitude and the mean stress. In contrast, a single Hammerstein model accounted for the data at all strain amplitudes and operating stresses. A Hammerstein model featuring a fifth-order polynomial static nonlinearity and a linear impulse response function of 1 s duration accounted for the most output variance (99.84% ± 0.13%, mean ± standard deviations for perturbations of 50% strain at 1.5 kPa stress). The static nonlinear behavior of the Hammerstein model also matched the quasistatic stress–strain behavior obtained at the same strain amplitude and operating stress. These results show that the static nonlinear behavior of the dog lung tissue strip is separable from its linear dynamic behavior. © 1998 Biomedical Engineering Society.
PAC98: 8745Bp, 8710+e
Similar content being viewed by others
REFERENCES
Barnas, G. M., D. Stamenovic, K. R. Lutchen, and C. F. Mackenzie. Lung and chest wall impedances in the dog: Effects of frequency and tidal volume. J. Appl. Physiol. 72:87-93, 1992.
2 Bates, J. H. T., G. N. Maksym, D. Navajas, and B. Suki. Lung tissue rheology and 1/f noise. Ann. Biomed. Eng. 22:674-681, 1994.
Dechman, G. S., D. A. Chartrand, P. P. Ruiz-Neto, and J. H. T. Bates. The effect of changing end-expiratory pressure on respiratory system mechanics in open-and closed-chest anesthetized, paralyzed patients. Anesth. Analg. 81:279-286, 1995.
Fredberg, J. J., D. Bunk, E. Ingenito, and S. A. Shore. Tissue resistance and the contractile state of lung parenchyma. J. Appl. Physiol. 74:1387-1397, 1993.
Fredberg, J. J., and D. Stamenovic. On the imperfect elasticity of lung tissue. J. Appl. Physiol. 67:2408-2419, 1989.
Fukaya, H., C. J. Martin, A. C. Young, and S. Katsura. Mechanical properties of alveolar walls. J. Appl. Physiol. 25:689-695, 1968.
Fung, Y. C. Biomechanics, Mechanical Properties of Living Tissues. New York: Springer-Verlag, 1981, p. 336.
Hantos, Z., B. Daroczy, T. Csendes, B. Suki, and S. Nagy. Modeling of low-frequency pulmonary impedance in dogs. J. Appl. Physiol. 68:849-860, 1990.
Hildebrandt, J. Pressure-volume data of cat lung interpreted by a plastoelastic, linear viscoelastic model. J. Appl. Physiol. 28:365-372, 1970.
Hines, W. H., and D. C. Montgomery. Probability and Statistics in Engineering and Management Science. 3rd ed., Toronto: Wiley, 1990, pp. 569-575.
Hunter, I. W., and R. E. Kearney. Generation of random sequences with jointly specified probability density and autocorrelation functions. Biol. Cybern. 47:141-146, 1983.
Hunter, I. W., and R. E. Kearney. Two-sided linear filter identification. Med. Biol. Eng. Comp. 21:203-209, 1983.
Hunter, I. W., and M. J. Korenberg. The identification of nonlinear biological systems: Wiener and Hammerstein cascade models. Biol. Cybern. 55:135-144, 1986.
Korenberg, M. J., and I. W. Hunter. Identification of nonlinear biological systems: Volterra kernel approaches. Ann. Biomed. Eng. 24:250-268, 1996.
Lee, G. C., and A. Frankus. Elasticity properties of lung parenchyma derived from experimental distortion data. Biophys. J. 15:481-493, 1975.
Maksym, G. N., and J. H. T. Bates. Nonparametric block structured modeling of rat lung mechanics. Ann. Biomed. Eng. (in press).
Marmarelis, P. Z., and V. Z. Marmarelis. Analysis of Physiologic Systems. New York: Plenum, 1978.
Mijailovich, S. M., D. Stamenovic, and J. J. Fredberg. Toward a kinetic theory of connective tissue micromechanics. J. Appl. Physiol. 74:665-681, 1993.
Mijailovich, S. M., D. Stamenovic, R. Brown, D. E. Lieth, and J. J. Fredberg. Dynamic moduli of rabbit lung tissue and pigeon ligamentum propatagiale undergoing uniaxial cyclic loading. J. Appl. Physiol. 76:773-782, 1994.
Moretto, A., M. Dallaire, P. Romero, and M. Ludwig. Effect of elastase on oscillation mechanics of lung parenchymal strips. J. Appl. Physiol. 77:1623-1629, 1994.
Navajas, D., G. N. Maksym, and J. H. T. Bates. Dynamic viscoelastic nonlinearity of lung parenchymal tissue. J. Appl. Physiol. 79:348-356, 1995.
Radford, E. P. Recent studies of the mechanical properties of mammalian lungs. In: Tissue Elasticity, edited by J. W. Remington. Washington, DC: Am. Physiol. Soc., 1957, pp. 177-190.
Stamenovic, D., G. M. Glass, G. M. Barnas, and J. J. Fredberg. Viscoplasticity of respiratory tissues. J. Appl. Physiol. 69:973-988, 1990.
Stamenovic, D., K. R. Lutchen, and G. M. Barnas. Alternative model of respiratory tissue viscoplasticity. J. Appl. Physiol. 75:1062-1069, 1993.
Sugihara, T., C. J. Martin, and J. Hildebrandt. Length-tension properties of alveolar wall in man. J. Appl. Physiol. 30:874-878, 1971.
Suki, B. Nonlinear phenomena in respiratory mechanical measurements. J. Appl. Physiol. 74:2574-2584, 1993.
Suki, B., A. L. Barabasi, and K. R. Lutchen. Lung tissue viscoelasticity: A mathematical framework and its molecular basis. J. Appl. Physiol. 76:2749-2759, 1994.
Suki, B., and J. H. T. Bates. A nonlinear viscoelastic model of lung tissue mechanics. J. Appl. Physiol. 71:826-833, 1991.
Suki, B., Z. Hantos, B. Daroczy, G. Alkaysi, and S. Nagy. Nonlinearity and harmonic distortion of dog lungs measured by low-frequency forced oscillations. J. Appl. Physiol. 71:69-75, 1991.
Suki, B., Q. Zhang, and K. R. Lutchen. Relationship between frequency and amplitude dependence in the lung: A nonlinear block-structured modeling approach. J. Appl. Physiol. 79:660-671, 1995.
Suwa, N., H. Fukasawa, R. Fujimoto, and M. Kawakami. Strain and stress of pulmonary tissues. Tohoku J. Exp. Med. 90:61-75, 1966.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maksym, G.N., Kearney, R.E. & Bates, J.H.T. Nonparametric Block-Structured Modeling of Lung Tissue Strip Mechanics. Annals of Biomedical Engineering 26, 242–252 (1998). https://doi.org/10.1114/1.119
Issue Date:
DOI: https://doi.org/10.1114/1.119