Abstract
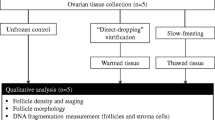
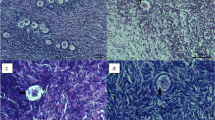
During cryopreservation of ovarian tissue, the conditions of freezing and thawing are big factors controlling the survival rate of oocytes obtained. However, the conditions and procedures as they pertain to ovarian follicles and oocytes have not been established. Thus, we tried to determine the appropriate freeze — thaw times using the vitrification method with ethylene glycol and DMSO as cryoprotective agents and dd Y female mouse ovaries. The maturity rate from GV to the metaphase-II (MII) stage was 62.8% with ethylene glycol and 69.3% using DMSO, while the controls (GV oocytes obtained from a fresh ovary) showed a maturation rate of 83.6% (46/55). MII oocytes obtained by culturing GV oocytes in vitro showed a 64.3% (18/28) fertility rate via in vitro fertilization and a developmental rate into a 2 cell stage embryo of 35.7% (10/28) and into a 4-cell stage, 7.1% (2/28). However, development beyond the 8 cell stage embryo was not observed.
A significant difference was not recognized between control (fresh) and ovarian tissues that had been frozen/thawed with respect to their ability to produce hormones.
It is concluded that the vitrification method was effective for both freezing ovarian tissues and preserving its functional ability (maturation and capacitation).
Similar content being viewed by others
References
Brachet A: Development in vitro de blastomeres et jeunes embryos demammiferes. C. R. Acad. Sci. (Paris) 155: 1191, 1912
McLaren A and Biggers JD: Successful development and birth of mice cultivated in vitro as early embryos. Nature 182: 877–878, 1958.
Ogawa S, Sato K, Hamada M, Hashimoto H: Culture of rabbit blastocyst in a medium with a convection current Nature 238: 402–405, 1972.
Ishiwata I, Tokieda Y, Ishiwata C, Okane N, Iguchi M, Sato K, Ishikawa H: Effects of feeder cells (human cancer cell lines) on the development of mouse embryos by co-culture. Human Cell 10: 237–246, 1997.
Ishiwata I, Tokieda Y, Kiguchi K, Sato K, Ishikawa H: Effects of embryotrophic factors on the embryogenesis and organogenesis of mouse embryos in vitro. Human Cell 13: 185–195, 2000.
Austin CR: Fertilization of mammalian eggs in vitro. Inter. Rev. Cytol. 12: 337–359, 1961.
Polge C and Rowson LEA: Fertilization capacity of bull spermatozoa after freezing at-7°C, Nature 169: 626–627, 1952.
Whittingham DG: Survival of mouse embryos after freezing and thawing, Nature 233: 125, 1971.
Wilmut I. and Rowson, LEA: Experiments on the low-temperature preservation of cow embryos. Vet. Rec. 92: 686–690, 1973.
Hovatta O, Silye R, Abir R, et al: Extracellular matrix improves of both sstored and fresh human primordial and primary ovarian follicles in longterm culture. Hum Reprod, 12: 1032–1036, 1977.
Abir R, Moore FT, Franks S, et al: Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril, 68: 682–688, 1997
Eppig JJ: Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod. Fertil. Dev., 8: 485–489, 1996.
Kito S and Bavister BD: Gonadotropin, serum, and amino acids after nuclear maturation, cumulus expansion, and oocyte morphology in hamster cumulus-oocyte complexes in vitro. Biol. Reprod., 56: 1281–1289, 1997.
De La Feunte, R, O’Brien, M.J., and Eppig, J.H.: Epidermal Growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Human Reprod., 14: 3060–3068, 1999.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tokieda, Y., Ishiwata, I., Segino, M. et al. Establishment of a novel method for cryopreservation and thawing of the mouse ovary. Hum Cell 15, 230–237 (2002). https://doi.org/10.1111/j.1749-0774.2002.tb00120.x
Published:
Issue Date:
DOI: https://doi.org/10.1111/j.1749-0774.2002.tb00120.x