Abstract
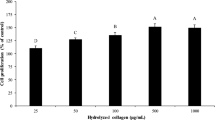
Previous observations showed that scallop shells contain organic components that have various useful applications for skin. In this study, the effect of the organic components of scallop shell (scallop shell extract) on collagen metabolism is investigated. Collagen metabolism is tightly controlled by the collagen degrading matrix metalloproteinases (MMP) and their tissue inhibitors (TIMP). Treatment of human skin fibroblast cells with the scallop shell extract increased the mRNA expression levels of type I collagen, MMP-1 and TIMP-1, suggesting that the scallop shell extract may activate collagen metabolism in skin fibroblast cells. Sirius red staining and the colorimetric quantification of collagen in fibroblast cells demonstrated that the scallop shell extract increased collagen content by approximately 1.3-fold. In vivo studies also revealed that the topical application of the scallop shell extract to rat dorsal skin increased the collagen content in the skin tissue section. These results suggest that the scallop shell extract may be effective for the treatment of photoaged and aging skin, which undergo collagen loss.
Similar content being viewed by others
References
Liu YC, Torita A, Hasegawa Y. Scallop shell extract promotes recovery from UV-B-induced damage in rat skin epidermal layer. Fish. Sci. 2006; 72: 388–392.
Liu YC, Uchiyama K, Natsui N, Hasegawa Y. In vitro activities of the components from scallop shells. Fish. Sci. 2002; 68: 1330–1336.
Torita A, Liu YC, Hasegawa Y. Photoprotective activity in rat skin keratinocyte of the scallop shell extract. Fish. Sci. 2004; 70: 910–915.
Liu YC, Torita A, Hasegawa Y. Scallop shell extract inhibits squalene monohydroperoxide-induced skin erythema and wrinkle formation in rat. Fish. Sci. (in press).
Oishi Y, Fu ZW, Ohnuki Y, Kato H, Noguchi T. Molecular basis of the alteration in skin collagen metabolism in response to in vivo dexamethasone treatment: effects on the synthesis of collagen type I and III, collagenase, and tissue inhibitors of metalloproteinases. Br. J. Dermatol. 2002; 147: 859–868.
Epstein EH Jr. [α1(III)]3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J. Biol. Chem. 1974; 249: 3225–3231.
Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J. Invest. Dermatol. 2003; 120: 842–848.
Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002; 138: 1462–1470.
Jenkins G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002; 123: 801–810.
Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002; 1: 705–720.
Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, Kang S, Voorhees JJ. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J. Clin. Invest. 2000; 106: 663–670.
Manthorpe M, Fagnani R, Skaper SD, Varon S. An automated colorimetric microassay for neurotrophic factors. Brain Res. 1986; 390: 191–198.
Tullberg-Reinert H, Jundt G. In situ measurement of collagen synthesis by human bone cells with a Sirius Redbased colorimetric microassay: effects of transforming growth factor β2 and ascorbic acid 2-phosphate. Histochem. Cell Biol. 1999; 112: 271–276.
Leon AL, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffinembedded sections. J. Histochem. Cytochem. 1985; 33: 737–743.
Esteban FJ, Moral ML, Sánchez-López AM, Blanco S, Jiménez A, Hernández R, Pedrosa JA, Peinado MA. Colorimetric quantification and in situ detection of collagen. J. Biol. Educ. 2005; 39: 183–186.
Srumpf M, Cao W, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V. Increased distribution of collagen type III and reduced expression of matrix metalloproteinase 1 in patients with diverticular disease. Int. J. Colorectal Dis. 2001; 16: 271–275.
Junge K, Rosch R, Anurov M, Titkova S, Ottinger A, Klinge U, Schumpelick V. Modification of collagen formation using supplemented mesh materials. Hernia 2006; 10: 492–497.
Joseph J, Kennedy RH, Devi S, Wang J, Joseph L, Hauer-Jensen M. Protective role of mast cells in homocysteine-induced cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 2005; 288: H2541-H2545.
Sanai A, Nagata H, Konno A. Extensive interstitial collagen deposition on the basement membrane zone in allergic nasal mucosa. Acta Otolaryngol. 1999; 119: 473–478.
Okano Y, Obayashi K, Yahagi S, Kurihara S, Kaburagi S, Kurata Y, Masaki H. Improvement of wrinkles by an all-trans-retinoic acid derivative, D-δ-tocopheryl retinoate. J. Dermatol. Sci. 2006; 2 (Suppl.): S65-S74.
Schröder JM. Cytokine networks in the skin. J. Invest. Dermatol. 1995; 105: 20S-24S.
Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol. Med. 2005; 117: 69–80.
Nawrat P, Surazynski A, Karna E, Palka JA. The effect of hyaluronic acid on interleukin-1-induced deregulation of collagen metabolism in cultured human skin fibroblasts. Pharmacol. Res. 2005; 51: 473–477.
Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J. Cell. Sci. 1999; 112: 1843–1853.
Griffiths CE, Voorhees JJ. Topical retinoic acid for photoaging: clinical response and underlying mechanisms. Skin Pharmacol. 1993; 6 (Suppl. 1): 70–77.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torita, A., Miyamoto, A. & Hasegawa, Y. The effects of scallop shell extract on collagen synthesis. Fish Sci 73, 1388–1394 (2007). https://doi.org/10.1111/j.1444-2906.2007.01482.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1444-2906.2007.01482.x