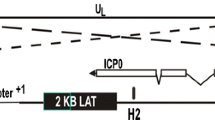
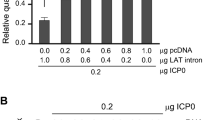
Abstract
The replication of herpes simplex virus (HSV) in epithelial cells, and during reactivation from latency in sensory neurons, depends on a ubiquitous cellular protein called host cell factor (HCF). The HSV transactivator, VP16, which initiates the viral replicative cycle, binds HCF as do some other cellular proteins. Of these, the neuronal transcription factor Zhangfei suppresses the ability of VP16 to initiate the replicative cycle. It also suppresses Luman, another cellular transcription factor that binds HCF. Interactions of nerve growth factor (NGF) and its receptor tropomyosin-related kinase (trkA) appear to be critical for maintaining HSV latency. Because the neuronal transcription factor Brn3a, which regulates trkA expression, has a motif for binding HCF, we investigated if Zhangfei had an effect on its activity. We found that Brn3a required HCF for activating the trkA promoter and Zhangfei suppressed its activity in non-neuronal cells. However, in neuron-like NGF-differentiated PC12 cells, both Brn3a and Zhangfei activated the trkA promoter and induced the expression of endogenous trkA. In addition, capsaicin, a stressor, which activates HSV in in vitro models of latency, decreased levels of Zhangfei and trkA transcripts in NGF-differentiated PC12 cells.
Similar content being viewed by others
References
Akhova O, Bainbridge M, Misra V (2005). The neuronal host cell factor-binding protein Zhangfei inhibits herpes simplex virus replication. J Virol 79: 14708–14718.
Andersen PL, Zhou H, Pastushok L, Moraes T, McKenna S, Ziola B, Ellison MJ, Dixit VM, Xiao W (2005). Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. J Cell Biol 170: 745–755.
Batchvarova N, Wang XZ, Ron D (1995). Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153). EMBO J 14: 4654–4661.
Bearer EL, Satpute-Krishnan P (2002). The role of the cytoskeleton in the life cycle of viruses and intracellular bacteria: tracks, motors, and polymerization machines. Curr Drug Targets Infect Disord 2: 247–264.
Bibel M, Barde YA (2000). Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14: 2919–2937.
Block T, Barney S, Masonis J, Maggioncalda J, Valyi-Nagy T, Fraser NW (1994). Long term herpes simplex virus type 1 infection of nerve growth factor-treated PC12 cells. J Gen Virol 75(Pt 9): 2481–2487.
Chen BP, Wolfgang CD, Hai T (1996). Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol 16: 1157–1168.
Chen CA, Okayama H (1988). Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques 6: 632–638.
Colgin MA, Smith RL, Wilcox CL (2001). Inducible cyclic AMP early repressor produces reactivation of latent herpes simplex virus type 1 in neurons in vitro. J Virol 75: 2912–2920.
Efstathiou S, Preston CM (2005). Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res 111: 108–119.
Freiman RN, Herr W (1997). Viral mimicry: common mode of association with HCF by VP16 and the cellular protein LZIP. Genes Dev 11: 3122–3127.
Ganju P, O’Bryan JP, Der C, Winter J, James IF (1998). Differential regulation of SHC proteins by nerve growth factor in sensory neurons and PC12 cells. Eur J Neurosci 10: 1995–2008.
Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM (2002). The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol 76: 12813–12822.
Geenen K, Nauwynck HJ, De Regge N, Braeckmans K, Favoreel HW (2007). Brn-3a suppresses pseudorabies virus-induced cell death in sensory neurons. J Gen Virol 88: 743–747.
Gupta A, Gartner JJ, Sethupathy P, Hatzigeorgiou AG, Fraser NW (2006). Anti-apoptotic function of a micro-RNA encoded by the HSV-1 latency-associated transcript. Nature 442: 82–85.
Hill JM, Garza HHJ, Helmy MF, Cook SD, Osborne PA, Johnson EMJ, Thompson HW, Green LC, O’Callaghan RJ, Gebhardt BM (1997). Nerve growth factor antibody stimulates reactivation of ocular herpes simplex virus type 1 in latently infected rabbits. J NeuroVirol 3: 206–211.
Hogan MR, Cockram GP, Lu R (2006). Cooperative interaction of Zhangfei and ATF4 in transactivation of the cyclic AMP response element. FEBS Lett 580: 58–62.
Huang EJ, Reichardt LF (2003). Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72: 609–642.
Hunsperger EA, Wilcox CL (2003). Capsaicin-induced reactivation of latent herpes simplex virus type 1 in sensory neurons in culture. J Gen Virol 84: 1071–1078.
Izumiya Y, Lin SF, Ellison T, Chen LY, Izumiya C, Luciw P, Kung HJ (2003a). Kaposi’s sarcoma-associated herpes-virus K-bZIP is a coregulator of K-Rta: physical association and promoter-dependent transcriptional repression. J Virol 77: 1441–1451.
Izumiya Y, Lin SF, Ellison TJ, Levy AM, Mayeur GL, Izumiya C, Kung HJ (2003b). Cell cycle regulation by Kaposi’s sarcoma-associated herpesvirus K-bZIP: direct interaction with cyclin-CDK2 and induction of G1 growth arrest. J Virol 77: 9652–9661.
Jin L, Peng W, Perng GC, Brick DJ, Nesburn AB, Jones C, Wechsler SL (2003). Identification of herpes simplex virus type 1 latency-associated transcript sequences that both inhibit apoptosis and enhance the spontaneous reactivation phenotype. J Virol 77: 6556–6561.
Jones C (2003). Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin Microbiol Rev 16: 79–95.
Jordan R, Pepe J, Schaffer PA (1998). Characterization of a nerve growth factor-inducible cellular activity that enhances herpes simplex virus type 1 gene expression and replication of an ICP0 null mutant in cells of neural lineage. J Virol 72: 5373–5382.
Khurana B, Kristie TM (2004). A Protein Sequestering System Reveals Control of Cellular Programs by the Transcriptional Coactivator HCF-1. J Biol Chem 279: 33673–33683.
Kriesel JD (1999). Reactivation of herpes simplex virus: the role of cytokines and intracellular factors. Curr Opin Infect Dis 12: 235–238.
Kristie TM, Vogel JL, Sears AE (1999). Nuclear localization of the C1 factor (host cell factor) in sensory neurons correlates with reactivation of herpes simplex virus from latency. Proc Natl Acad Sci U S A 96: 1229–1233.
LaBoissiere S, Hughes T, O’Hare P (1999). HCF-dependent nuclear import of VP16. EMBO J 18: 480–489.
Laycock KA, Brady RH, Lee SF, Osborne PA, Johnson EM, Pepose JS (1994). The role of nerve growth factor in modulating herpes simplex virus reactivation in vivo. Graefes Arch Clin Exp Ophthalmol 232: 421–425.
Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, Kokame K, Lu R (2006). Luman/CREB3 induces transcription of the endoplasmic reticulum stress response protein, Herp, through an ERSE-II element. Mol Cell Biol 26: 7999–8010.
Liao W, Tang Y, Lin SF, Kung HJ, Giam CZ (2003). K-bZIP of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) binds KSHV/HHV-8 Rta and represses Rta-mediated transactivation. J Virol 77: 3809–3815.
Lu R, Misra V (2000a). Potential role for Luman, the cellular homologue of herpes simplex virus VP16 (alpha gene trans-inducing factor), in herpesvirus latency. J Virol 74: 934–943.
Lu R, Misra V (2000b). Zhangfei: a second cellular protein interacts with herpes simplex virus accessory factor HCF in a manner similar to Luman and VP16. Nucleic Acids Res 28: 2446–2454.
Lu R, Yang P, O’Hare P, Misra V (1997). Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol 17: 5117–5126.
Lu R, Yang P, Padmakumar S, Misra V (1998). The herpesvirus transactivator VP16 mimics a human basic domain leucine zipper protein, Luman, in its interaction with HCF. J Virol 72: 6291–6297.
Luciano RL, Wilson AC (2003). HCF-1 functions as a coactivator for the zinc finger protein Krox20. J Biol Chem 278: 51116–51124.
Luxton GW, Haverlock S, Coller KE, Antinone SE, Pincetic A, Smith GA (2005). Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc Natl Acad Sci U S A 102: 5832–5837.
Ma L, Lei L, Eng SR, Turner E, Parada LF (2003). Brn3a regulation of TrkA/NGF receptor expression in developing sensory neurons. Development 130: 3525–3534.
Ma L, Merenmies J, Parada LF (2000). Molecular characterization of the TrkA/NGF receptor minimal enhancer reveals regulation by multiple cis elements to drive embryonic neuron expression. Development 127: 3777–3788.
Misra V, Rapin N, Akhova O, Bainbridge M, Korchinski P (2005). Zhangfei is a potent and specific inhibitor of the host cell factor-binding transcription factor Luman. J Biol Chem 280: 15257–15266.
Misra V, Walker S, Hayes S, O’Hare P (1995). The bovine herpesvirus alpha gene trans-inducing factor activates transcription by mechanisms different from those of its herpes simplex virus type 1 counterpart VP16. JVirol 69: 5209–5216.
Narayanan A, Nogueira ML, Ruyechan WT, Kristie TM (2005). Combinatorial transcription of herpes simplex virus and varicella zoster virus immediate early genes is strictly determined by the cellular coactivator HCF-1. J Biol Chem 280: 1369–1375.
Narayanan A, Ruyechan WT, Kristie TM (2007). The coactivator host cell factor-1 mediates Set1 and MLL1 H3K4 trimethylation at herpesvirus immediate early promoters for initiation of infection. Proc Natl Acad Sci U S A 104: 10835–10840.
Parada LF, Tsoulfas P, Tessarollo L, Blair J, Reid SW, Soppet D (1992). The Trk family of tyrosine kinases: receptors for NGF-related neurotrophins. Cold Spring Harbor Symp Quant Biol 57: 43–51.
Preston CM (2000). Repression of viral transcription during herpes simplex virus latency. J Gen Virol 81(Pt 1): 1–19.
Qiao S, Li W, Tsubouchi R, Murakami K, Yoshino M (2004). Role of vanilloid receptors in the capsaicin-mediated induction of iNOS in PC12 cells. Neurochem Res 29: 687–693.
Raggo C, Rapin N, Stirling J, Gobeil P, Smith-Windsor E, O’Hare P, Misra V (2002). Luman, the cellular counterpart of herpes simplex virus VP16, is processed by regulated intramembrane proteolysis. Mol Cell Biol 22: 5639–5649.
Roizman B, Knipe DM (2001). Herpes simplex viruses and their replication. In Field’s Virology. Knipe DM, Howley PM, Griffin DE, Martin MA, Lamb RA, Roizman B, Straus SE (ed). Lippincott Williams & Wilkins, Philadelphia. Pp 2399–2460
Shirakawa K, Maeda S, Gotoh T, Hayashi M, Shinomiya K, Ehata S, Nishimura R, Mori M, Onozaki K, Hayashi H, Uematsu S, Akira S, Ogata E, Miyazono K, Imamura T (2006). CCAAT/enhancer-binding protein homologous protein (CHOP) regulates osteoblast differentiation. Mol Cell Biol 26: 6105–6116.
Smith RL, Pizer LI, Johnson EMJ, Wilcox CL (1992). Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology 188: 311–318.
Sok J, Wang XZ, Batchvarova N, Kuroda M, Harding H, Ron D (1999). CHOP-Dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol 19: 495–504.
Someya A, Kunieda K, Akiyama N, Hirabayashi T, Horie S, Murayama T (2004). Expression of vanilloid VR1 receptor in PC12 cells. Neurochem Int 45: 1005–1010.
Suzuki H, Ogawa C, Usui K, Hayashizaki Y (2004). In vitro pull-down assay without expression constructs. Biotechniques 37: 918, 920.
Thompson RL, Sawtell NM (2001). Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J Virol 75: 6660–6675.
Tyagi S, Chabes AL, Wysocka J, Herr W (2007). E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol Cell 27: 107–119.
Ubeda M, Habener JF (2003). CHOP transcription factor phosphorylation by casein kinase 2 inhibits transcriptional activation. J Biol Chem 278: 40514–40520.
Ubeda M, Vallejo M, Habener JF (1999). CHOP enhancement of gene transcription by interactions with Jun/Fos AP-1 complex proteins. Mol Cell Biol 19: 7589–7599.
Ubeda M, Wang XZ, Zinszner H, Wu I, Habener JF, Ron D (1996). Stress-induced binding of the transcriptional factor CHOP to a novel DNA control element. Mol Cell Biol 16: 1479–1489.
Valderrama X, Misra V (2008). Novel Brn3a cis-acting sequences mediate transcription of human trkA in neurons. J Neurochem 105: 425–435.
Wilcox C, Johnson E (1987). Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J Virol 61: 2311–2315.
Wilcox C, Johnson E (1988). Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol 62: 393–399.
Wilcox CL, Smith RL, Freed CR, Johnson EMJ (1990). Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci 10: 1268–1275.
Wysocka J, Herr W (2003). The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem Sci 28: 294–304.
Yu JY, DeRuiter SL, Turner DL (2002). RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A 99: 6047–6052.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a Discovery Grant to V.M. from the Natural Sciences and Engineering Research Council of Canada. X.V. was supported partially by the Discovery grant and partially by a University of Saskatchewan graduate scholarship.
Rights and permissions
About this article
Cite this article
Valderrama, X., Rapin, N. & Misra, V. Zhangfei, a novel regulator of the human nerve growth factor receptor, trkA. Journal of NeuroVirology 14, 425–436 (2008). https://doi.org/10.1080/13550280802275904
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/13550280802275904