Abstract
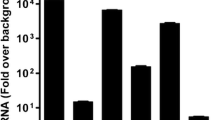
On entering sensory ganglia, herpes simplex viruses 1 (HSV-1) establishes a latent infection with the synthesis of a latency associated transcript (LAT) or initiates productive infection with expression of a set of immediate early viral proteins. The precise mechanisms how expression of α genes is suppressed during the latency are unknown. One mechanism that has been proposed is illustrated in the case of ICP0, a key immediate early viral regulatory protein. Specifically, the 2 kb LAT intron is complementary to the 3′ terminal portion of ICP0 mRNA. To test the hypothesis that accumulation of LAT negatively affects the accumulation of ICP0 mRNA, we inserted a DNA fragment encoding two poly(A) sequences into LAT to early terminate LAT transcript without interrupting the complementary sequence of ICP0 transcript (named as SR1603). Comparisons of the parent (SR1601) and mutant (SR1603) HSV-1 viruses showed the following: Neurons harboring latent SR1603 virus accumulated equivalent amounts of viral DNA but higher amounts of ICP0 mRNA and lower amounts of LAT, when compared to neurons harboring the SR1601 virus. One notable difference between the two viruses is that viral RNA accumulation in explanted ganglia harboring SR1603 virus initiated significantly sooner than that in neurons harboring SR1601 virus, suggesting that ICP0 may act as an activator of viral gene expression in permissive cells. Collectively, these data suggest that increased ICP0 mRNA by suppressed LAT did not affect the establishment of latency in latently infected murine ganglia.







Similar content being viewed by others
References
Burton EA, Hong CS, Glorioso JC (2003) The stable 2.0-kilobase intron of the herpes simplex virus type 1 latency-associated transcript does not function as an antisense repressor of ICP0 in nonneuronal cells. J Virol 77:3516–3530
Cai W, Schaffer PA (1992) Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol 66:2904–2915
Chen SH, Lee LY, Garber DA, Schaffer PA, Knipe DM, Coen DM (2002) Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J Virol 76:4764–4772
Devi-Rao GB, Goodart SA, Hecht LM, Rochford R, Rice MK, Wagner EK (1991) Relationship between polyadenylated and nonpolyadenylated herpes simplex virus type 1 latency-associated transcripts. J Virol 65:2179–2190
Du T, Han Z, Zhou G, Roizman B (2015) Patterns of accumulation of miRNAs encoded by herpes simplex virus during productive infection, latency, and on reactivation. Proc Natl Acad Sci USA 112:E49–55
Du T, Zhou G, Roizman B (2011) HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs. Proc Natl Acad Sci USA 108:18820–18824
Du T, Zhou G, Roizman B (2012) Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc Natl Acad Sci USA 109:14616–14621
Farrell MJ, Dobson AT, Feldman LT (1991) Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci USA 88:790–794
Garber DA, Schaffer PA, Knipe DM (1997) A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J Virol 71:5885–5893
Guo J, Garrett M, Micklem G, Brogna S (2011) Poly(A) signals located near the 5' end of genes are silenced by a general mechanism that prevents premature 3'-end processing. Mol Cell Biol 31:639–651
Ho DY, Mocarski ES (1989) Herpes simplex virus latent RNA (LAT) is not required for latent infection in the mouse. Proc Natl Acad Sci USA 86:7596–7600
Honess RW, Roizman B (1975) Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA 72:1276–1280
Horsburgh BC, Hubinette MM, Tufaro F (1999) Genetic manipulation of herpes simplex virus using bacterial artificial chromosomes. Methods Enzymol 306:337–352
Huang R, Wu J, Zhou X, Jiang H, Guoying Zhou G, Roizman B (2019) Herpes simplex virus 1 MicroRNA miR-H28 exported to uninfected cells in exosomes restricts cell-to-cell virus spread by inducing gamma interferon mRNA. J Virol 93:e01005-19
Mador N, Goldenberg D, Cohen O, Panet A, Steiner I (1998) Herpes simplex virus type 1 latency-associated transcripts suppress viral replication and reduce immediate-early gene mRNA levels in a neuronal cell line. J Virol 72:5067–5075
Nahmias AJ, Roizman B (1973) Infection with herpes-simplex viruses 1 and 2. N Engl J Med 289:781–789
Nicoll MP, Hann W, Shivkumar M, Harman LE, Connor V, Coleman HM, Proenca JT, Efstathiou S (2016) The HSV-1 latency-associated transcript functions to repress latent phase lytic gene expression and suppress virus reactivation from latently infected neurons. PLoS Pathog 12:e1005539
Nicosia M, Deshmane SL, Zabolotny JM, Valyi-Nagy T, Fraser NW (1993) Herpes simplex virus type 1 latency-associated transcript (LAT) promoter deletion mutants can express a 2-kilobase transcript mapping to the LAT region. J Virol 67:7276–7283
Roizman B (1979) The organization of the herpes simplex virus genomes. Annu Rev Genet 13:25–57
Roizman B, Sears AE (1987) An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol 41:543–571
Sears AE, Meignier B, Roizman B (1985) Establishment of latency in mice by herpes simplex virus 1 recombinants that carry insertions affecting regulation of the thymidine kinase gene. J Virol 55:410–416
Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT (1987) RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056–1059
Wagner EK, Devi-Rao G, Feldman LT, Dobson AT, Zhang YF, Flanagan WM, Stevens JG (1988) Physical characterization of the herpes simplex virus latency-associated transcript in neurons. J Virol 62:1194–1202
Wahle E (1995) 3'-end cleavage and polyadenylation of mRNA precursors. Biochim Biophys Acta 1261:183–194
Yan R, Zhou X, Chen X, Liu X, Ma J, Wang L, Liu Z, Zhan B, Chen H, Wang J, Zou W, Xu H, Lu R, Ni D, Roizman B, Zhou GG (2019) Enhancement of oncolytic activity of oHSV expressing IL-12 and anti PD-1 antibody by concurrent administration of exosomes carrying CTLA-4 miRNA. Immunotherapy 5:154
Zhou G, Du T, Roizman B (2013a) HSV carrying WT REST establishes latency but reactivates only if the synthesis of REST is suppressed. Proc Natl Acad Sci USA 110:E498–506
Zhou G, Du T, Roizman B (2013b) The role of the CoREST/REST repressor complex in herpes simplex virus 1 productive infection and in latency. Viruses 5:1208–1218
Zhou G, Ye GJ, Debinski W, Roizman B (2002) Engineered herpes simplex virus 1 is dependent on IL13Ralpha 2 receptor for cell entry and independent of glycoprotein D receptor interaction. Proc Natl Acad Sci USA 99:15124–15129
Acknowledgements
These studies were supported by grants from Shenzhen Overseas High-Caliber Peacock Foundation KQTD2015071414385495, Shenzhen Science and Innovation Commission Project Grants JCYJ20180306173333907 to Shenzhen International Institute for Biomedical Research.
Author information
Authors and Affiliations
Contributions
HJ, JW, XL, GZ, WF designed research; HJ, JW, XL, RL, MZ, MC, YL performed research; HJ, JW, GZ, WF analyzed data, GZ, WF wrote and finalized the paper. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no conflict of interests.
Animal and Human Rights Statement
All animal studies were done in accordance with guidelines and protocols approved by the Institutional Animal Care and Use Committee of the Shenzhen International Institute for Biomedical Research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, H., Wu, J., Liu, X. et al. Termination of Transcription of LAT Increases the Amounts of ICP0 mRNA but Does Not Alter the Course of HSV-1 Infection in Latently Infected Murine Ganglia. Virol. Sin. 36, 264–272 (2021). https://doi.org/10.1007/s12250-020-00287-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-020-00287-2