Summary
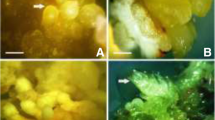
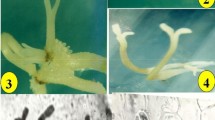
In vitro propagation of Quassia amara L. (Simaroubaceae) was attempted using mature and juvenile explants. Attempts to establish in vitro culture using leaf and internode explants from a plant more than 15yr old were unsuccessful due to severe phenolic exudation. Plant regeneration through direct and indirect somatic embryogenesis was established from cotyledon explants. Murashige and Skoog (MS) medium with 8.9 μM N6-benzyladenine (BA) and 11.7 μM silver nitrate induced the highest number (mean of 32.4 embryos per cotyledon) of somatic embryos. Direct somatic embryogenesis as well as callus formation was observed on medium with BA (8.9–13.3 μM). Semi-mature pale green cotyledons were superior for the induction of somatic embryos. Embryos developed from the adaxial side as well as from the point of excision of the embryonic axis. More embryos were developed on the proximal end compared to mid and distal regions of the cotyledons. Subculture of callus (developed along with the somatic embryos on medium with BA alone) onto medium containing 8.9 μM BA and 11.7 μM silver nitrate produced a mean of 17.1 somatic embryos. Primary somatic embryos cultured on MS medium with 8.9 μM BA and 11.7μM silver nitrate produced a mean of 9.4 secondary somatic embryos. Most of the embryos developed up to early cotyledonary stage. Reduced concentration of BA (2.2 or 4.4 μM) improved maturation and conversion of embryos to plantlets. Ninety percent of the embryos converted to plantlets. The optimized protocol facilitated recovery of 30 plantlets per cotyledon explant within 80d. Plantlets transferred to small cups were subsequently transferred to field conditions with a survival rate of 90%.
Similar content being viewed by others
References
Ainsley, P. J.; Hammerschlag, F. A.; Bertozzi, T.; Collins, G. G.; Sedgley, M. Regeneration of almond from immature seed cotyledons. Plant Cell Tiss. Organ Cult. 67:221–226; 2001.
Anonymous. The Wealth of India. Raw materials, vol. VIII. New Delhi: CSIR; 1969:345.
Barbetti, P.; Grandolini, G.; Fardella, G.; Chiappini, I. Indole alkaloids from Quassia amara. Planta Med. 53:289–290; 1987.
Cherry, G. Can organic methods be used to produce quality top fruit? New Farmer and Grower (Autumn):22–24; 1991.
Choi, Y.-E.; Yang, D.-C.; Yoon, E.-S.; Choi, K.-T. Plant regeneration via adventitious bud formation from cotyledon explants of Panax ginseng C.A. Meyer. Plant Cell Rep. 17:731–736; 1998.
Crosby, D. G. Minor insecticides of plant origin. In: Jacobsen, M.; Crosby, D. G., eds. Naturally occurring insecticides. New York: Dekker; 1971:177–239.
Duncan, D. B. Multiple range and multiple F-tests. Biometrics 11:1–42; 1955.
Fiore, M. C.; Trabace, T.; Sunseri, F. High frequency of plant regeneration in sunflower from cotyledons via somatic embryogenesis. Plant Cell Rep. 16:295–298; 1997.
Kupchan, S. M.; Streelman, D. R. Quassimarin, a new antileukemic quassinoid from Quassia amara. J. Org. Chem. 41:3481–3482; 1976.
Mandal, A. K. A.; Gupta, S. D. Somatic embryogenesis of safflower: influence of auxin and ontogeny of somatic embryos. Plant Cell Tiss. Organ Cult. 72:27–32; 2003.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays for tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Rai, V. R.; McComb, J. Direct somatic embryogenesis from mature embryos of sandalwood. Plant Cell Tiss. Organ Cult. 69:65–70; 2002.
Rastogi, R. P.; Mehrotra, B.N. Compendium of Indian medicinal plants, vol. 1 1960–1969. New Delhi: CDRI and Publication; 1993:336.
Rout, G. R.; Das, P. Somatic embryogenesis from callus cultures of Simarouba glauca Linn. Indian J. Exp. Biol. 32:582–583; 1994.
Scragg, A. H.; Allan, E. J. Picrasma quassinoides Bennett: in vitro culture and production of quassin. In: Bajaj, Y. P. S., ed Biotechnology and agriculture and forestry, vol. 21. Medicinal and aromatic plants IV. Berlin: Springer-Verlag; 1992:249–268.
Scragg, A. H.; Allan, E. J. Quassia amara (Surinam Quassia): in vitro culture and the production of quassin. In: Bajaj, Y. P. S., ed. Biotechnology and agriculture and forestry, vol. 26. Medicinal and aromatic plants VI. Berlin: Springer-Verlag; 1994:316–326.
Scragg, A. H.; Ashton, S.; Steward, R. D.; Allan, E. J. Growth of and quassin accumulation by cultures of Quassia amara. Plant Cell Tiss. Organ Cult. 23:165–169; 1990.
Singh, A. K.; Chand, S.; Pattnaik, S.; Chand, P. K. Adventitious shoot organogenesis and plant regeneration from cotyledons of Dalbergia sissoo Roxb., a timber yielding tree legume. Plant Cell Tiss. Organ Cult. 68:203–209; 2002.
Venkatachalam, P.; Geetha, N.; Khandelwal, A.; Shaila, M. S.; Sita, G. L. Induction of direct somatic embryogenesis and plant regeneration from mature cotyledons explants of Arachis hypogaea L. Curr. Sci. 77:269–273; 1999.
Vikrant, A.; Rashid, A. Somatic embryogenesis from immature and mature embryos of a minor millet Paspalum scrobiculatum. L. Plant Cell Tiss. Organ Cult. 69:71–77; 2002.
Welander, M. Plant regeneration from leaf and stem segments of shoots raised in vitro from mature apple trees. J. Plant Physiol. 132:738–744; 1988.
Zhang, P.; Phansiri, S.; Puonti-Kaerlas, J. Improvement of cassava shoot organogenesis by the use of silver nitrate in vitro. Plant Cell Tiss. Organ Cult. 67:47–54; 2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, K.P., Madassery, J. Direct and indirect somatic embryogenesis on cotyledon explants of Quassia amara L., an antileukaemic drug plant. In Vitro Cell.Dev.Biol.-Plant 41, 54–57 (2005). https://doi.org/10.1079/IVP2004588
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2004588