Summary
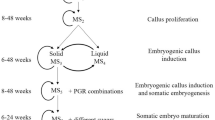
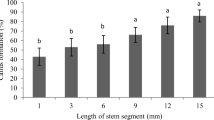
The diploid cotton species can constitute a valuable gene pool for the more agronomically desirable cultivated tetraploid cultivars and offer better opportunities to study gene structure and function through gene knockouts. In order to exploit these advantages, a regeneration system is required to achieve these transformation-based goals. Carbohydrate source and concentration were evaluated to improve somatic embryo (SE) production and desiccation treatments to improve the conversion efficiency of SEs to plants in a diploid Gossypium arboreum accession, A2-9 (PI-529712). Improved SE numbers and their subsequent conversion into plantlets was achieved with a Murashige and Skoog (MS)/sucrose-based medium M2 [0.04M sucrose, 0.3 μM α-naphthaleneacetic acid (NAA)] On this medium, 219 embryos per g initiated, and close to 11% of these embryos germinated into plantlets. Neither a 5-d desiccation treatment of embryogenic callus previously cultured in liquid medium nor filter paper insertion improved the numbers of SEs induced or their conversion to plantlets. A 3-d desiccation period resulted in improved plant regeneration. When immature G. arboreum SEs induced on M1 (0.2M glucose, 2.6 μM NAA, and 0.2 μM kinetin) medium underwent a 3-d desiccation treatment, 49% of these immature SEs were converted to plantlets after a 4-wk period on M2 medium. These improved results will help to pave the way for future genetic transformation and associated gene structure and function studies utilizing G. arboreum. These results, in particular the 3-d desiccation treatment, can also be incorporated into regeneration protocols to improve the regeneration efficiency of other Gossypium species.
Similar content being viewed by others
References
Blanc, G.; Michaux-Ferriere, N.; Teisson, C.; Lardet, L.; Carron, M. P. Effects of carbohydrate addition on the induction of somatic embryogenesis in Hevea brasiliensis. Plant. Cell Tiss. Organ Cult. 59:103–112; 1999.
Blank, L. M. Southwestern cotton rust. In: Brown, J. M., ed. Proc. Beltwide Cotton Conf., Atlanta, GA, Jannary 12–13, Memphis, TN: National Cotton Council; 1971: 76–77.
Bomal, C.; Tremblay, F. M. Effect of desiecation to low moisture content on germination, synchronization of root emergence, and plantlet regeneration of black spruce somatic embryos. Plant. Cell Tiss. Organ Cult. 56:193–200; 1999.
Choudhary, B.; Laroia, G. Technological development and cotton production in India and China. Curr. Sci. 80:925–932; 2001.
Davidonis, G. H.; Hamilton, R. H. Plant regeneration from callus tissue of Gossypium hirsutum L. Plant Sci. Lett. 32:89–93; 1983.
Dhawan, A. K.; Simwat, G. S.; Siddhu, A. S. Field reaction of some varieties of Asiatic cotton (Gossypium arboreum L.) to sucking and bollworm pests. J. Res. Punjab Agr. Univ. 28:57–62; 1991.
Dogra, C. S.; Desi cotton comes to rescue farmers. The Hindustan Times. September 5. 1998. http://www.hindustantimes.com/nonfram/ 050998/detECO06.htm
Finer, J. J. Plant regeneration from somatic embryogenic suspension cultures of cotton (Gossypium hirsutum L.). Plant Cell Rep. 7:399–402; 1988.
Firoozabady, E.; DeBoer, D. L. Plant regeneration via somatic embryogenesis in many cultivars of cotton (Gossypium hirsutum L.). In Vitro Cell. Dev. Biol. Plant 299:166–173; 1993.
International Cotton Advisory Committee (ICAC). Cotton: its origin and areas of production. http://www.icac.org/icac/projects/commonfund/ cppnd/cppndehpl.pdf; 1994: 5–36.
Kazuko, Y. S.; Kazuko, S. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or highsalt stress. Plant Cell 6:251–264; 1994.
Ladyman, J. A. R.; Girard, B. Cucumber somatic embryo development on various gelling agents and carbohydrate sources. HortScience 27:164–165; 1992.
Lee, E. K.; Cho, Y. D.; Soh, W. Y. Effect of humidity on somatic embryogenesis in cotyledon explant culture of carrot. J. Plant Biol. 40:89–94; 1997.
Liu, W.; Hildebrand, D. F.; Moore, P. J.; Collins, G. B. Expression of desiccation-induced and lipoxygenase genes during the transition from the maturation to the germination phases in soybean somatic embryos. Planta 194:69–76; 1994.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Pack, K. Y.; Hahn, E.-J. Cytokinins, auxins and activated charcoal affect organogenesis and anatomical characteristics of shoot-tip cultures of Lisianthus [Eustoma grandiflorum (Raf.) Shinn]. In Vitro Cell. Dev. Biol. Plant 36:128–132; 2000.
Parrott, W. A.; Dryden, G.; Vogt, S.; Hildebrand, D. F.; Collins, G. B.; Williams, E. G. Optimization of somatic embryogenesis and embryo germination in soybean. In Vitro Cell. Dev. Biol. Plant 24:817–821; 1988.
Pomeroy, K.; Brown, D. C. W.; Takahata, Y. Response of Brassica napus L. microspore-derived embryos to exogenous abseisic acid and desiccation. In Vitro Cell. Dev. Biol. Plant. 30:196–203; 1994.
Rajasekaran, K.; Chlan, C. A.; Cleveland, T. E. Tissue culture and genetic transformation of cotton. In: Jenkins, J. N.; Saha, S., eds. Genetic improvement of cotton: emerging technologies, Enfield, NH: Science Publishing, Inc.; 2000:2700290.
Rajasekaran, K.; Mullius, M. G. Embryos and planflets from cultured anthers of hybrid grapevines. J. Exp. Bot. 30:399–407; 1979.
Ritala, A.; Mannonen, L.; Oksman-Caldentey, K. M. Factors affecting the regeneration capacity of isolated barley microspores (Hordeum vulgare L.). Plant Cell Rep. 20:403–407; 2001.
Sakhanokho, H. F.; Zipf. A.; Rajasekaran, K.; Saha, S.; Sharma, G. C. Sugar and nitrogen sources significantly increase somatic embryogenesis in diploid cotton. Gossypium arboreum. In: 2001 Proc. Plant Growth Regulation Society of America, July 1–5, Miami Beach, FL; 2001a: 82–87.
Sakhanokho, H. F.; Zipf, A. Rajasekaran, K.; Saha, S.; Sharma, G. C. Induction of highly embryogenic calli and plant regeneration in Upland and Pima cottons. Crop. Sci. 41:1235–1240; 2001b.
SAS Institute. SAS systems for linear modell release 8.2. Cary, NC: SAS Institute, Inc. 1999.
Shoemaker, R. C.; Couche, L. J.; Galbraith, D. W.; Characterization of somatic embryogenesis and plant regeneration in cotton (Gossypium hirsutum). Plant Cell Rep. 3:178–181; 1986.
Smith, R. H.; Price, H. J.; Thaxton, J. B. Defined conditions for the initiation and growth of cotton callus in vitro. I. Gossypium arboreum. In Vitro 13:329–334; 1977.
Sunilkumar, G.; Rathore, K. S. Transgenic cotton: factors influencing Agrobacterium-mediated transformation and regeneration. Mol. Breed. 8:37–52; 2001.
Teng, W. L. Activated charcoal affects morphogenesis and enhances sporophyte regeneration during leaf cell suspension culture of Platycerium bifurcutum. Plant Cell Rep. 17:77–83; 1997.
Timbert, R.; Barbotin, J. N.; Thomas, D. Enhancing carrot somatic embryo survival during slow dehydration, by encapsulation and control of dehydration. Plant Sci. 120:215–222; 1996.
Trolinder, N. L.; Xhixian, C. Genotype specificity of the somatic embryogenesis response in cotton. Plant Cell Rep. 8:133–136; 1989.
Whecler, T. A.; Gannaway, J. R.; Keating, K. Identification of resistance to Thielaviopsis basicola in diploid species. Plant Dis. 83:831–833; 1999.
Yehoshua, S.; Rhodes, D.; Janick, J. Changes in amino acid composition associated with tolerance to partial desiccation of celery somatic embryos. J. Am. Soc. Hort. Sci. 117:337–341; 1992.
Zimmerman, J. L.; Apuya, N.; Darwish, K. O.; Carroll, P. Novel regulation of heat shock genes during carrot somatic embryo development. Plant Cell 12:1137–1146; 1989.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakhanokho, H.F., Zipf, A., Rajasekaran, K. et al. Somatic embryo initiation and germination in diploid cotton (Gossypium arboreum L.). In Vitro Cell.Dev.Biol.-Plant 40, 177–181 (2004). https://doi.org/10.1079/IVP2003497
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2003497