Abstract
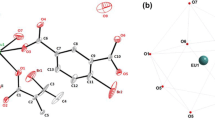
The photophysical properties of Eu3+ and Tb3+ complexes of DOTAGA and DO3A-monoamide conjugates of the Pittsburgh compound B (PiB) chromophore, prepared using linkers of different lengths and flexibilities, and which form stable negatively charged (LnL1), and uncharged (LnL2) complexes, respectively, were studied as potential probes for optical detection of amyloid aggregates. The phenylbenzothiazole (PiB) moiety absorbs light at wavelengths longer than 330 nm with a high molar absorption coefficient in both probes, and acts as an antenna in these systems. The presence of the luminescent Ln3+ ion quenches the excited states of PiB through an energy transfer process from the triplet state of PiB to the metal centre, and structured emission is seen from Eu3+ and Tb3+. The luminescence study indicates the presence of a 5D4 → T1 back transfer process in the Tb3+ complexes. It also provides insights on structural properties of the Eu3+ complexes, such as the high symmetry environment of the Eu3+ ion in a single macrocyclic conformation and the presence of one water molecule in its inner coordination sphere. The overall quantum yield of luminescence of EuL1 is higher than for EuL2. However, their low values reflect the low overall sensitization efficiency of the energy transfer process, which is a consequence of the large distances between the metal center and the antenna, especially in the EuL2 complex. DFT calculations confirmed that the most stable conformation of the Eu3+ complexes involves a combination of a square antiprismatic (SAP) geometry of the chelate and an extended conformation of the linker. The large calculated average distances between the metal center and the antenna point to the predominance of the Förster energy transfer mechanism, especially for EuL2. This study provides insights into the behavior of amyloid-targeted Ln3+ complexes as optical probes, and contributes towards their rational design.
Similar content being viewed by others
Notes and references
M. B. Graeber, S. Kösel, R. Egensperger, R. B. Banati, U. Müller, K. Bise, P. Hoff, H. J. Möller, K. Fujisawa and P. Mehraein, Rediscovery of the case described by Alois Alzheimer in 1911: Historical, histological and molecular genetic analysis, Neurogenetics 1997 1 73
G. Macchi, C. Brahe and M. Pomponi, Alois Alzheimer and Gaetano Perusini: should man divide what fate united?, Behav. Neurol. 1997 4 210
D. Galimberti and E. Scarpini, Progress in Alzheimer’s Disease, J. Neurol. 2012 259 201
J. A. Hardy and G. A. Higgins, Alzheimer’s disease: the amyloid cascade hypothesis, Science 1992 256 184
R. Roychaudhuri, M. Yang, M. M. Hoshi and D. B. Teplow, Amyloid beta-protein assembly and Alzheimer disease, J. Biol. Chem. 2009 284 4749
J. Hardy and D. J. Selkoe, The Amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics, Science 2002 297 353
K. Rajasekhar, M. Chakrabarti and T. Govindaraju, Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer’s disease, Chem. Commun. 2015 51 13434
R. Sherrington, E. I. Rogaev, Y. Liang, E. A. Rogaeva, G. Levesque, M. Ikeda, H. Chi, C. Lin, G. Li, K. Holman, T. Tsuda, L. Mar, J. F. Foncin, A. C. Bruni, M. P. Montesi, S. Sorbi, I. Rainero, L. Pinessi, L. Nee, I. Chumakov, D. Pollen, A. Brookes, P. Sanseau, R. J. Polinsky, W. Wasco, H. A. R. Da Silva, J. L. Haines, M. A. Perkicak-Vance, R. E. Tanzi, A. D. Roses, P. E. Fraser, J. M. Rommens and P. H. George-Hyslop, Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease, Nature 1995 375 754
E. Levy-Lahad, W. Wasco, P. Poorkaj, D. M. Romano, J. Oshima, W. H. Pettingell, C. Yu, P. D. Jondro, S. D. Schmidt, K. Wang, A. C. Crowley, Y. Fu, S. Y. Guenette, D. Galas, E. Nemens, E. M. Wijsman, T. D. Bird, G. D. Schellenberg and R. E. Tanzi, Candidate gene for the chromosome 1 familial Alzheimer’s disease locus, Science 1995 269 973
B. Dubois, H. H. Feldman, C. Jacova, S. T. Dekosky, P. Barberger-Gateau, J. Cummings, A. Delacourte, D. Galasko, S. Gauthier, G. Jicha, K. Meguro, J. O′brien, F. Pasquier, P. Robert, M. Rossor, S. Salloway, Y. Stern, P. J. Visser and P. Scheltens, Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria, Lancet Neurol. 2007 6 734
W. M. Pardridge, The blood-brain barrier: bottleneck in brain drug development, NeuroRx 2005 2 3
C. A. Lipinski, Lead-and drug-like compounds: the rule-of-five revolution, Drug Discovery Today: Technol. 2004 1 337
D. Balériaux, C. Colosimo, J. Ruscalleda, M. Korves, G. Schneider, K. Bohndorf, G. Bongartz, M. A. Buchem, M. Reiser, K. Sartor, M. W. Bourne, P. M. Parizel, G. R. Cherryman, I. Salerio, L. A. Noce, G. Pirovano, M. A. Kirchin and A. Spinazzi, Magnetic resonance imaging of metastatic disease to the brain with gadobenate dimeglumine, Neuroradiology 2002 44 191
A. Petiet and M. Dhenain, Improvement of microscopic MR imaging of amyloid plaques with targeting and non-targeting contrast agents, Curr. Med. Imaging Rev. 2011 7 8
C. A. Mathis, B. J. Bacskai, S. T. Kajdasz, M. E. McLellan, M. P. Frosch, B. T. Hyman, D. P. Holt, Y. Wang, G.-F. Huang, M. L. Debnath and W. E. Klunk, A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain, Bioorg. Med. Chem. Lett. 2002 12 295
M.-P. Kung, C. Hou, Z.-P. Zhuang, D. Skovronsky and H. F. Kung, Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissues of patients with Alzheimer’s disease, Brain Res. 2004 1025 98
R. Vandenberghe, K. Van Laere, A. Ivanoiu, E. Salmon, C. Bastin, E. Triau, S. Hasselbalch, I. Law, A. Andersen, A. Korner, L. Minthon, G. Garraux, N. Nelissen, G. Bormans, C. Buckley, R. Owenius, L. Thurfjell, G. Farrar and D. J. Brooks, 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: A phase 2 trial, Ann. Neurol. 2010 68 319
C. M. Clark, J. A. Schneider, B. J. Bedell, T. G. Beach, W. B. Bilker, M. A. Mintun, M. J. Pontecorvo, F. Hefti, A. P. Carpenter, M. L. Flitter, M. J. Krautkramer, H. F. Kung, R. E. Coleman, P. M. Doraiswamy, A. S. Fleisher, M. N. Sabbagh, C. H. Adowsky, E. M. Reiman, S. P. Zehntner and D. M. Skovronsky, Use of florbetapir-PET for imaging β-amyloid pathology, J. Am. Med. Assoc. 2011 305 275
E. Liu, M. E. Schmidt, R. Margolin, R. Koeppe, N. S. Mason, W. E. Klunk, C. A. Mathis, S. Salloway, N. C. Fox, D. L. Hill, A. S. Les, P. Collins, K. M. Gregg, J. Di, Y. Lu, I. C. Tudor, B. T. Wyman, K. Booth, E. Yuen and H. R. Brashear, Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials, Neurology 2015 85 692
W. E. Klunk and C. A. Mathis, The future of amyloid-beta imaging: a tale of radionuclides and tracer proliferation, Curr. Opin. Neurol. 2008 21 683–687
S. M. Landau, C. Breault, A. D. Joshi, M. Pontecorvo, C. A. Mathis, W. J. Jagust and M. A. Mintun, Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods, J. Nucl. Med. 2013 54 70
H. Watanabe, M. Ono, S. Iikuni, M. Yoshimura, K. Matsumura, H. Kimura and H. Saji, A (68)Ga complex based on benzofuran scaffold for the detection of β-amyloid plaques, Bioorg. Med. Chem. Lett. 2014 24 4834
S. Lacerda, J.-F. Morfin, C. F. G. C. Geraldes and É. Tóth, Metal complexes for multimodal imaging of misfolded protein-related diseases, Dalton Trans. 2017 46 14461
K. Chauhan, A. Datta, A. Adhikari, K. Chuttani, A. K. Singh and A. K. Mishra, 68Ga based probe for Alzheimer’s disease: synthesis and preclinical evaluation of homodimeric chalcone in β-amyloid imaging, Org. Biomol. Chem. 2014 12 7328
M. Asti, E. Ferrari, S. Croci, G. Atti, S. Rubagotti, M. Iori, P. C. Capponi, A. Zerbini, M. Saladini and A. Versari, Synthesis and characterization of 68Ga-labeled curcumin and curcuminoid complexes as potential radiotracers for imaging of cancer and Alzheimer’s disease, Inorg. Chem. 2014 53 4922
D. Cressier, M. Dhilly, T. T. Cao Pham, F. Fillesoye, F. Gourand, A. Maïza, A. F. Martins, J.-F. Morfin, C. F. G. C. Geraldes, É. Tóth and L. Barré, Gallium-68 complexes conjugated to Pittsburgh compound B: radiolabeling and biological evaluation, Mol. Imaging Biol. 2016 18 334
D. J. Hayne, S. Lim and P. S. Donnelly, Metal complexes designed to bind to amyloid-β for the diagnosis and treatment of Alzheimer’s disease, Chem. Soc. Rev. 2014 43 6701
A. Forsberg, H. Engler, O. Almkvist, G. Blomquist, G. Hagman, A. Wall, A. Ringheim, B. Långström and A. Nordberg, PET imaging of amyloid deposition in patients with mild cognitive impairment, Neurobiol. Aging 2008 29 1456
X. Chen, P. Yu, L. Zhang and B. Liu, Synthesis and biological evaluation of 99mTc,Re-monoamine-monoamide conjugated to 2-(4-aminophenyl)benzothiazole as potential probes for β-amyloid plaques in the brain, Bioorg. Med. Chem. Lett. 2008 18 1442
Z.-P. Zhuang, M.-P. Kung, C. Hou, K. Ploessl and H. F. Kung, Biphenyls labeled with technetium 99 m for imaging beta-amyloid plaques in the brain, Nucl. Med. Biol. 2005 32 171
M. Ono, R. Ikeoka, H. Watanabe, H. Kimura, T. Fuchigami, M. Haratake, H. Saji and M. Nakayama, Synthesis and evaluation of novel chalcone derivatives with (99 m)Tc/Re complexes as potential probes for detection of β-amyloid plaques, ACS Chem. Neurosci. 2010 1 598
J. F. Poduslo, G. L. Curran, J. A. Peterson, D. J. McCormick, A. H. Fauq, M. A. Khan and T. M. Wengenack, Design and chemical synthesis of a Magnetic Resonance contrast agent with enhanced in vitro binding, high blood-brain barrier permeability, and in vivo targeting to Alzheimer’s disease amyloid plaques, Biochemistry 2004 43 6064
Y. Z. Wadghiri, E. M. Sigurdsson, M. Sadowski, J. I. Elliott, Y. Li, H. Scholtzova, C. Y. Tang, G. Aguinaldo, M. Pappolla, K. Duff, T. Wisniewski and D. H. Turnbull, Detection of Alzheimer’s amyloid in transgenic mice using Magnetic Resonance Microimaging, Magn. Reson. Med. 2003 50 293
J. F. Poduslo, T. M. Wengenack, G. L. Curran, T. Wisniewski, E. M. Sigurdsson, S. I. Macura, B. J. Borowski and C. R. Jack, Molecular targeting of Alzheimer’s amyloid plaques for contrast-enhanced Magnetic Resonance Imaging, Neurobiol. Dis. 2002 11 315
J. Yang, Y. Z. Wadghiri, D. M. Hoang, W. Tsui, Y. Sun, E. Chung, Y. Li, A. Wang, M. de Leon and T. Wisniewski, Detection of amyloid plaques targeted by USPIO-Aβ1-42 in Alzheimer’s disease transgenic mice using Magnetic Resonance Microimaging, Neuroimage 2011 55 1600
A. F. Martins, J.-F. Morfin, A. Kubíčková, V. Kubíček, F. Buron, F. Suzenet, M. Salerno, A. N. Lazar, C. Duyckaerts, N. Arlicot, D. Guilloteau, C. F. G. C. Geraldes and E. Tóth, PiB-conjugated, metal-based imaging probes: multimodal approaches for the visualization of β-amyloid plaques, ACS Med. Chem. Lett. 2013 4 436
A. F. Martins, J.-F. Morfin, C. F. G. C. Geraldes and E. Tóth, Gd3+ complexes conjugated to Pittsburgh compound B: potential MRI markers of b-amyloid plaques, J. Biol. Inorg. Chem. 2014 19 281
A. F. Martins, D. M. Dias, J.-F. Morfin, S. Lacerda, D. V. Laurents, É. Tóth and C. F. G. C. Geraldes, Interaction of PiB-derivative metal complexes with beta-amyloid peptides: selective recognition of the aggregated forms, Chem. - Eur. J. 2015 21 5413
G. Bort, S. Catoen, H. Borderies, A. Kebsi, S. Ballet, G. Louin, M. Port and C. Ferroud, Gadolinium-based contrast agents targeted to amyloid aggregates for the early diagnosis of Alzheimer’s disease by MRI, Eur. J. Med. Chem. 2014 87 843
W. E. Klunk, M. L. Debnath and J. W. Pettegrew, Chrysamine-G binding to Alzheimer and control brain: Autopsy study of a new amyloid probe, Neurobiol. Aging 1995 16 541
C. Zhu, L. Liu, Q. Yang, F. Lv and S. Wang, Water-soluble conjugated polymers for imaging, diagnosis, and therapy, Chem. Rev. 2012 112 4687
C. J. Sigurdson, K. P. R. Nilsson, S. Hornemann, G. Manco, M. Polymenidou, P. Schwarz, M. Leclerc, P. Hammarstro, K. Wüthrich and A. Aguzzi, Prion strain discrimination using luminescent conjugated polymers, Nat. Methods 2007 4 1023
E. E. Nesterov, J. Skoch, B. T. Hyman, W. E. Klunk, B. J. Bacskai and T. M. Swager, In vivo optical imaging of amyloid aggregates in brain: design of fluorescent markers, Angew. Chem., Int. Ed. 2005 44 5452
A. Åslund, C. J. Sigurdson, T. Klingstedt, S. Grathwohl, T. Bolmont, D. L. Dickstein, E. Glimsdal, S. Prokop, M. Lindgren, P. Konradsson, D. M. Holtzman, P. R. Hof, F. L. Heppner, S. Gandy, M. Jucker, A. Aguzzi, P. Hammarstro and K. P. R. Nilsson, Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses, ACS Chem. Biol. 2009 4 673
L. Civitelli, L. Sandin, E. Nelson, S. I. Khattak, A.-C. Brorsson and K. Kågedal, The luminescent oligothiophene p-FTAA converts toxic Aβ1-42 species into nontoxic amyloid fibers with altered properties, J. Biol. Chem. 2016 291 9233
N. P. Cook, V. Torres, D. Jain and A. A. Martí, Sensing amyloid-β aggregation using luminescent dipyridophenazine ruthenium(II) complexes, J. Am. Chem. Soc. 2011 133 11121
J.-C. G. Bünzli and S. V. Eliseeva, Intriguing aspects of lanthanide luminescence, Chem. Sci. 2013 4 1939
J.-C. G. Bünzli, Lanthanide luminescence for biomedical analyses and imaging, Chem. Rev. 2010 110 2729
D. Parker, Luminescent lanthanide sensors for pH, pO2 and selected anions, Coord. Chem. Rev. 2000 205 109
H. Uh and S. Petoud, Novel antennae for the sensitization of near infrared luminescent lanthanide cations, C. R. Chim. 2010 13 668
S. Faulkner, S. J. A. Pope, B. P. Burton-Pye, Lanthanide complexes for luminescence imaging applications, Appl. Spectrosc. Rev. 2005 40 1
A. M. Smith, M. C. Mancini and S. Nie, Bioimaging: Second window for in vivo imaging, Nat. Nanotechnol. 2009 4 710
I. Hemmilä and V. Laitala, Progress in lanthanides as luminescent probes, J. Fluoresc. 2005 15 529
A. F. Martins, A. C. Oliveira, J.-F. Morfin, D. V. Laurents, É. Tóth and C. F. G. C. Geraldes, Associating a negatively charged GdDOTA-derivative to the Pittsburgh compound B for targeting Aβ amyloid aggregates, J. Biol. Inorg. Chem. 2016 21 83
P. M. Costa, J. T.-W. Wang, J. F. Morfin, T. Khanum, W. To, J. Sosabowski, É. Tóth and K. T. Al-Jamal, Functionalised carbon nanotubes enhance brain delivery of amyloid-targeting Pittsburgh compound B (PiB)-derived ligands, Nanotheranostics 2018 2 168
A. D. Sherry, R. D. Brown III, C. F. G. C. Geraldes, S. H. Koenig, K.-T. Kuan and M. Spiller, Synthesis and characterization of the gadolinium(3+) complex of DOTA-propylamide: a model DOTA-protein conjugate, Inorg. Chem. 1989 620 1989
J. P. André, E. Brücher, R. Kiraly, R. A. Carvalho, H. Mäcke and C. F. G. C. Geraldes, DOTASA, an asymmetrical derivative of DOTA substituted at one acetate pendant arm: 1H NMR and potentiometric studies of the ligand and its lanthanide(III) complexes, Helv. Chim. Acta 2005 88 633
E. Brücher, G. Tircsó, Z. Baranyai, Z. Kovács and A. D. Sherry, in The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, ed. A. Merbach, L. Helm and É. Tóth, Wiley, Chichester, U.K., 2013, ch. 4, p. 157
A. Barge, G. Cravotto, E. Gianolio and F. Fedeli, How to determine free Gd and free ligand in solution of Gd chelates. A technical note, Contrast Media Mol. Imaging 2006 1 184
D. F. Evans, The determination of the paramagnetic susceptibility of substances in solution by nuclear magnetic resonance, J. Chem. Soc. 1959 2003
D. M. Corsi, C. Platas-Iglesias, H. Van Bekkum and J. A. Peters, Determination of paramagnetic lanthanide(III) concentrations from bulk magnetic susceptibility shifts in NMR spectra, Magn. Reson. Chem. 2001 39 723
J. N. Demas and G. A. Crosby, The measurement of photoluminescence quantum yields. A review, J. Phys. Chem. 1971 75 991
W. H. Melhuish, Quantum efficiencies of fluorescence of organic substances: effect of solvent and concentration of the fluorescent solute, J. Phys. Chem. 1961 65 229
K. Nakamaru, Synthesis, luminescence quantum yields, and lifetimes of trischelated ruthenium(II) mixed-ligand complexes including 3,3′-dimethyl-2,2′-bipyridyl, Bull, Chem. Soc. Jpn. 1982 55 2697
J. S. de Melo, J. Pina, F. B. Dias and A. L. Maçanita, in Applied Photochemistry, ed. R. C. Evans, P. Douglas and H. D. Burrows, Springer, 2013, ch. 15, p. 533
J. Pina, J. S. de Melo, H. D. Burrows, A. L. Maçanita, F. Galbrecht, T. Bünnagel and U. Scherf, Alternating binaphthyl-thiophene copolymers: synthesis, spectroscopy, and photophysics and their relevance to the question of energy migration versus conformational relaxation, Macromolecules 2009 42 1710
J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids, Phys. Rev. Lett. 2003 91 146401
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis and J. A. Montgomery, General atomic and molecular electronic structure system, J. Comput. Chem. 1993 14 1347
M. Dolg, H. Stoll, A. Savin and H. Preuss, Energy-adjusted pseudopotentials for the rare earth elements, Theor. Chim. Acta 1989 75 173
M. Dolg, H. Stoll and H. Preuss, A combination of quasi-relativistic pseudopotential and ligand field calculations for lanthanoid compounds, Theor. Chim. Acta 1993 85 441
A. F. Martins, S. V. Eliseeva, H. F. Carvalho, J. M. C. Teixeira, C. T. B. Paula, P. Hermann, C. Platas-Iglesias, S. Petoud, E. Tóth and C. F. G. C. Geraldes, A bis(pyridine N -oxide) analogue of DOTA: relaxometric properties of the GdIII complex and efficient sensitization of visible and NIR-emitting lanthanide(III) cations including PrIII and HoIII, Chem. - Eur. J. 2014 20 14834
F. Lu, R. Hu, S. Wang, X. Guo and G. Yang, Luminescent properties of benzothiazole derivatives and their application in white light emission, RSC Adv. 2017 7 4196
V. Jacques and J. F. Desreux, Quantitative two-dimensional EXSY spectroscopy and dynamic behavior of a paramagnetic lanthanide macrocyclic chelate: YbDOTA (DOTA = 1,4,7,10-Tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic Acid), Inorg. Chem. 1994 33 4048
J. C. P. Grancho, M. M. Pereira, M. da G. Miguel, A. M. Rocha Gonsalves and H. D. Burrows, Synthesis, spectra and photophysics of some free base tetrafluoroalkyl and tetrafluoroaryl porphyrins with potential applications in imaging, Photochem. Photobiol. 2002 75 249
P. A. Tanner, Some misconceptions concerning the electronic spectra of tri-positive europium and cerium, Chem. Soc. Rev. 2013 42 5090
K. Binnemans, Interpretation of europium(III) spectra, Coord. Chem. Rev. 2015 295 1
S. Aime, M. Botta, M. Fasano, M. P. M. Marques, C. F. G. C. Geraldes, D. Pubanz and A. E. Merbach, Conformational and coordination equilibria on DOTA complexes of lanthanide metal ions in aqueous solution studied by (1)H-NMR Spectroscopy, Inorg. Chem. 1997 36 2059
J. A. Peters, K. Djanashvili, C. F. G. C. Geraldes and C. Platas-Iglesias, in The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, ed. A. Merbach, L. Helm and É. Tóth, Wiley, 2nd edn, 2013, p. 209
M. Latva, H. Takalo, V.-M. Mukkala, C. Matachescu, J. C. Rodriguez-Ubis and J. Kankare, Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield, J. Lumin. 1997 75 149
M. Momtaldi, A. Credi, L. Prodi and M. T. Gandolfini, Handbook of Photochemistry, Taylor & Francis, N. Y., 2006
O. L. Malta, Mechanisms of non-radiative energy transfer involving lanthanide ions revisited, J. Non-Cryst. Solids 2008 354 4770
G. F. de Sá, O. L. Malta, C. de Mello Donegá, A. M. Simas, R. L. Longo, P. A. Santa-Cruz and E. F. da Silva Jr., Spectroscopic properties and design of highly luminescent lanthanide coordination complexes, Coord. Chem. Rev. 2000 196 165
A. N. C. Neto, E. E. S. Teotónio, G. F. de Sá, H. F. Brito, J. Legendziewicz, L. D. Carlos, M. C. F. C. Felinto, P. Gawryszewska, R. T. Moura Jr., R. L. Longo, W. M. Faustino and O. L. Malta, in Handbook on the Physics and Chemistry of Rare Earths, ed. J.-C. G. Bünzli and V. K. Pecharsky, Elsevier B.V., Amsterdam, The Netherlands, 2019, vol. 56, p. 55
S. Hassoon, H. Lustig, M. B. Rubin and S. Speiser, The mechanism of short-range intramolecular electronic energy transfer in bichromophoric molecules, J. Phys. Chem. 1984 88 6367
S. V. Eliseeva and J.-C. G. Bünzli, Lanthanide luminescence for functional materials and bio-sciences, Chem. Soc. Rev. 2010 39 189
J.-C. G. Bünzli, Lanthanide light for biology and medical diagnosis, J. Lumin. 2016 170 866
M. H. V. Werts, R. T. F. Jukes and J. W. Verhoeven, The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes, Phys. Chem. Chem. Phys. 2002 4 1542
A. Beeby, L. M. Bushby, D. Maffeo and G. J. A. Williams, Intramolecular sensitisation of lanthanide(III) luminescence by acetophenone-containing ligands: the critical effect of para-substituents and solvent, J. Chem. Soc., Dalton Trans. 2002 48
S. Quici, M. Cavazzini, G. Marzanni, G. Accorsi, N. Armaroli, B. Ventura and F. Barigelletti, Visible and Near-Infrared Intense Luminescence from Water-Soluble Lanthanide [Tb(III), Eu(III), Sm(III), Dy(III), Pr(III), Ho(III), Yb(III), Nd(III), Er(III)] Complexes, Inorg. Chem. 2005 44 529
W. D. Horrocks and D. R. Sudnick, Lanthanide ion luminescence probes of the structure of biological macromolecules, Acc. Chem. Res. 1981 14 384
A. Beeby, I. M. Clarkson, R. S. Dickins, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. G. Williams and M. Woods, Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states, J. Chem. Soc., Perkin Trans. 2 1999 493
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Oliveira, A.C., Costa, T., Justino, L.L.G. et al. Photophysical studies on lanthanide(iii) chelates conjugated to Pittsburgh compound B as luminescent probes targeted to Aβ amyloid aggregates. Photochem Photobiol Sci 19, 1522–1537 (2020). https://doi.org/10.1039/d0pp00214c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/d0pp00214c