Abstract
Photodynamic therapy (PDT) is a well-established treatment option in the treatment of certain cancerous and pre-cancerous lesions. Though best-known for its application in tumor therapy, historically the photodynamic effect was first demonstrated against bacteria at the beginning of the 20th century. Today, in light of spreading antibiotic resistance and the rise of new infections, this photodynamic inactivation (PDI) of microbes, such as bacteria, fungi, and viruses, is gaining considerable attention. This review focuses on the PDI of viruses as an alternative treatment in antiviral therapy, but also as a means of viral decontamination, covering mainly the literature of the last decade. The PDI of viruses shares the general action mechanism of photodynamic applications: the irradiation of a dye with light and the subsequent generation of reactive oxygen species (ROS) which are the effective phototoxic agents damaging virus targets by reacting with viral nucleic acids, lipids and proteins. Interestingly, a light-independent antiviral activity has also been found for some of these dyes. This review covers the compound classes employed in the PDI of viruses and their various areas of use. In the medical area, currently two fields stand out in which the PDI of viruses has found broader application: the purification of blood products and the treatment of human papilloma virus manifestations. However, the PDI of viruses has also found interest in such diverse areas as water and surface decontamination, and biosafety.
Similar content being viewed by others
Abbreviations
- AdV:
-
Adenovirus
- ALA:
-
δ-Aminolevulinic acid
- BoHV:
-
Bovine herpes virus
- BVDV:
-
Bovine viral diarrhea virus
- Cox:
-
Coxsackie virus
- DENV:
-
Dengue virus
- EBV:
-
Epstein Barr virus
- EIAV:
-
Equine infectious anemia virus
- EMCV:
-
Encephalomyocarditis virus
- FCV:
-
Feline calivirus
- FHV:
-
Feline herpes virus
- FVC:
-
Feline corona virus
- HA:
-
Haemagglutinin
- HAL:
-
Hexaaminolaevulinate
- HAV:
-
Hepatitis A virus
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- HMME:
-
Haematoporphyrin monomethyl ether
- HPD:
-
Haematoporphyrin derivative
- HPV:
-
Human papilloma virus
- HSV:
-
Herpes simplex virus
- IAV:
-
Influenza A virus
- IFN:
-
Interferon
- ISC:
-
Intersystem crossing
- JEV:
-
Japanese encephalitis virus
- MAL:
-
Methyl aminolevulinate
- MIV:
-
Mosquito iridovirus
- MNV:
-
Murine norovirus
- NDV:
-
Newcastle disease virus
- NIR:
-
Near infrared
- NP:
-
Nanoparticle
- NV:
-
Norovirus
- SFV:
-
Semliki Forest virus
- SOD:
-
Superoxide dismutase
- PACT:
-
Photodynamic antimicrobial chemotherapy
- PCI:
-
Photochemical internalization
- PDI:
-
Photodynamic inactivation
- PDT:
-
Photodynamic therapy
- PEG:
-
Polyethylene glycol
- PEI:
-
Polyethyleneimine
- PpIX:
-
Protoporphyrin IX
- PS:
-
Photosensitizer
- PVP:
-
Polyvinylpyrrolidone
- ROS:
-
Reactive oxygen species
- RV:
-
Rhinovirus
- SFV:
-
Semliki forest virus
- TNF:
-
Tumor necrosis factor
- UCNP:
-
Up-conversion nanoparticles
- VEEV:
-
Venezuelan equine encephalitis virus
- VSV:
-
Vesicular stomatitis virus
- ZnPc:
-
(Phthalocyaninato)zinc(II)
Notes and references
T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, Photodynamic Therapy, J. Natl. Cancer Inst., 1998, 90, 889–905.
T. N. Demidova and M. R. Hamblin, Photodynamic therapy targeted to pathogens, Int. J. Immunopathol. Pharmacol., 2004, 17, 245–254; (b) G. Jori and S. B. Brown, Photosensitized inactivation of microorganisms, Photochem. Photobiol. Sci., 2004, 3, 403–405; (c) M. R. Hamblin and T. Hasan, Photodynamic therapy: A new antimicrobial approach to infectious disease?, Photochem. Photobiol. Sci., 2004, 3, 436–450; (d) G. B. Kharkwal, S. K. Sharma, Y.-Y. Huang, T. Dai and M. R. Hamblin, Photodynamic therapy for infections: Clinical applications, Lasers Surg. Med., 2011, 43, 755–767.
T. Maisch, A New Strategy to Destroy Antibiotic Resistant Microorganisms: Antimicrobial Photodynamic Treatment, Mini-Rev. Med. Chem., 2009, 9, 974–983.
M. Kielmann, C. Prior and M. O. Senge, Porphyrins in troubled times: a spotlight on porphyrins and their metal complexes for explosives testing and CBRN defense, New J. Chem., 2018, 42, 7529–7550.
T. Maisch, R.-M. Szeimies, G. Jori and C. Abels, Antibacterial photodynamic therapy in dermatology, Photochem. Photobiol. Sci., 2004, 3, 907–917.
R. F. Donnelly, P. A. McCarron and M. M. Tunney, Antifungal photodynamic therapy, Microbiol. Res., 2008, 163, 1–12; (b) P. Calzavara-Pinton, M. T. Rossi, R. Sala and M. Venturini, Photodynamic Antifungal Chemotherapy, Photochem. Photobiol., 2012, 88, 512–522.
M. Wainwright, Photodynamic antimicrobial chemotherapy (PACT), J. Antimicrob. Chemother., 1998, 42, 13–28; (b) F. Cieplik, D. Deng, W. Crielaard, W. Buchalla, E. Hellwig, A. Al-Ahmad and T. Maisch, Antimicrobial photodynamic therapy – what we know and what we don’t, Crit. Rev. Microbiol., 2018, 44, 571–589.
L. Sobotta, P. Skupin-Mrugalska, J. Mielcarek, T. Goslinski and J. Balzarini, Photosensitizers Mediated Photodynamic Inactivation Against Virus Particles, Mini-Rev. Med. Chem., 2015, 15, 503–521; (b) O. Janouskova, J. Rakusan, M. Karaskova and K. Holada, Photodynamic inactivation of prions by disulfonated hydroxyaluminium phthalocyanine, J. Gen. Virol., 2012, 93, 2512–2517.
M. Wainwright, Photoinactivation of viruses, Photochem. Photobiol. Sci., 2004, 3, 406–411.
L. Costa, M. A. F. Faustino, M. G. P. M. S. Neves, Â. Cunha and A. Almeida, Photodynamic Inactivation of Mammalian Viruses and Bacteriophages, Viruses, 2012, 4, 1034–1074.
M. Wainwright, Local treatment of viral disease using photodynamic therapy, Int. J. Antimicrob. Agents, 2003, 21, 510–520.
L. Strasfeld and S. Chou, Antiviral Drug Resistance: Mechanisms and Clinical Implications, Infect. Dis. Clin. North Am., 2010, 24, 413–437; (b) K. K. Irwin, N. Renzette, T. F. Kowalik and J. D. Jensen, Antiviral drug resistance as an adaptive process, Virus Evol., 2016, 2, vew014, DOI: 10.1093/ve/vew014.
A. B. Ormond and H. S. Freeman, Dye Sensitizers for Photodynamic Therapy, Materials, 2013, 6, 817–840.
T. Maisch, Resistance in antimicrobial photodynamic inactivation of bacteria, Photochem. Photobiol. Sci., 2015, 14, 1518–1526.
M. Wainwright, T. Maisch, S. Nonell, K. Plaetzer, A. Almeida, G. P. Tegos and M. R. Hamblin, Photoantimicrobials—are we afraid of the light?, Lancet Infect. Dis., 2017, 17, e49–e55; (b) T. Dai, Y.-Y. Huang and M. R. Hamblin, Photodynamic therapy for localized infections – State of the art, Photodiagn. Photodyn. Ther., 2009, 6, 170–188.
C. d. P. Eduardo, A. C. C. Aranha, A. Simões, M. S. Bello-Silva, K. M. Ramalho, M. Esteves-Oliveira, P. M. de Freitas, J. Marotti and J. Tunér, Laser treatment of recurrent herpes labialis: a literature review, Lasers Med. Sci., 2014, 29, 1517–1529; (b) J. P. Kelley and R. M. Rashid, Phototherapy in the Treatment of Cutaneous Herpesvirus Manifestations, Cutis, 2011, 88, 140–148; (c) X. Wen, Y. Li and M. R. Hamblin, Photodynamic therapy in dermatology beyond non-melanoma cancer: An update, Photodiagn. Photodyn. Ther., 2017, 19, 140–152.
J. M. Dąbrowski and L. G. Arnaut, Photodynamic therapy (PDT) of cancer: from local to systemic treatment, Photochem. Photobiol. Sci., 2015, 14, 1765–1780; (b) M. Kwitniewski, A. Juzeniene, R. Glosnicka and J. Moan, Immunotherapy: a way to improve the therapeutic outcome of photodynamic therapy?, Photochem. Photobiol. Sci., 2008, 8, 1011–1017; (c) A. P. Castano, P. Mroz and M. R. Hamblin, Photodynamic therapy and anti-tumour immunity, Nat. Rev. Cancer, 2006, 6, 535–545.
E. Ben-Hur and R. P. Goodrich, Pathogen Reduction in Blood for Transfusion Using Photodynamic Treatments, in Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications, ed. M. R. Hamblin and G. Jori, RSC Publishing, Cambridge, UK, 2011, pp. 233–263; (b) P. Schlenke, Pathogen Inactivation Technologies for Cellular Blood Components: an Update, Transfus. Med. Hemother., 2014, 41, 309–325; (c) M. Lozano, J. Cid and T. H. Müller, Plasma Treated with Methylene Blue and Light: Clinical Efficacy and Safety Profile, Transfus. Med. Rev., 2013, 27, 235–240; (d) K. Schneider, L. Wronka-Edwards, M. Leggett-Embrey, E. Walker, P. Sun, B. Ondov, T. H. Wyman, M. J. Rosovitz, S. S. Bohn, J. Burans and T. Kochel, Psoralen Inactivation of Viruses: A Process for the Safe Manipulation of Viral Antigen and Nucleic Acid, Viruses, 2015, 7, 5875–5888; (e) C. L. Trimble and I. H. Frazer, Development of therapeutic HPV vaccines, Lancet Oncol., 2009, 10, 975–980.
C. Lange and P. J. Bednarski, Photosensitizers for Photodynamic Therapy: Photochemistry in the Service of Oncology, Curr. Pharm. Des., 2016, 22, 6956–6974; (b) D. van Straten, V. Mashayekhi, H. S. de Bruijn, S. Oliveira and D. J. Robinson, Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions, Cancers, 2017, 9, 19, DOI: 10.3390/cancers9020019; (c) P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Photodynamic Therapy of Cancer: An Update, Ca-Cancer J. Clin., 2011, 61, 250–281.
M. Q. Mesquita, C. J. Dias, M. G. P. M. S. Neves, A. Almeida and M. A. F. Faustino, Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy, Molecules, 2018, 23, 2424, DOI: 10.3390/molecules23102424; (b) H. Qiu, M. Tan, T. Y. Ohulchanskyy, J. F. Lovell and G. Chen, Recent Progress in Upconversion Photodynamic Therapy, Nanomaterials, 2018, 8, 344, DOI: 10.3390/nano8050344; (c) S. Perni, P. Prokopovich, J. Pratten, I. P. Parkin and M. Wilson, Nanoparticles: their potential use in antibacterial photodynamic therapy, Photochem. Photobiol. Sci., 2011, 10, 712–720; (d) D. Bechet, P. Couleaud, C. Frochot, M.-L. Viriot, F. Guillemin and M. Barberi-Heyob, Nanoparticles as vehicles for delivery of photodynamic therapy agents, Trends Biotechnol., 2008, 26, 612–621; (e) M. Sadasivam, P. Avci, G. K. Gupta, S. Lakshmanan, R. Chandran, Y.-Y. Huang, R. Kumar and M. R. Hamblin, Self-assembled liposomal nanoparticles in photodynamic therapy, Eur. J. Nanomed., 2013, 5, 115–129; (f) C. M. Cassidy, M. M. Tunney, P. A. McCarron and R. F. Donnelly, Drug delivery strategies for photodynamic antimicrobial chemotherapy: From benchtop to clinical practice, J. Photochem. Photobiol., B, 2009, 95, 71–80.
S. S. Lucky, K. C. Soo and Y. Zhang, Nanoparticles in Photodynamic Therapy, Chem. Rev., 2015, 115, 1990–2042; (b) D. K. Chatterjee, L. S. Fong and Y. Zhang, Nanoparticles in photodynamic therapy: An emerging paradigm, Adv. Drug Delivery Rev., 2008, 60, 1627–1637; (c) M. Slingerland, H.-J. Guchelaar and H. Gelderblom, Liposomal drug formulations in cancer therapy: 15 years along the road, Drug Discovery Today, 2012, 17, 160–166.
E. Paszko, C. Ehrhardt, M. O. Senge, D. P. Kelleher and J. V. Reynolds, Nanodrug applications in photodynamic therapy, Photodiagn. Photodyn. Ther., 2011, 8, 14–29; (b) G. Obaid, M. Broekgaarden, A.-L. Bulin, H.-C. Huang, J. Kuriakose, J. Liu and T. Hasan, Photonanomedicine: a convergence of photodynamic therapy and nanotechnology, Nanoscale, 2016, 8, 12471–12503.
R. G. van der Molen, J. Garssen, A. de Klerk, F. H. J. Claas, M. Norval, H. van Loveren, H. K. Koerten and A. M. Mommaas, Application of a systemic herpes simplex virus type 1 infection in the rat as a tool for sunscreen photoimmunoprotection studies, Photochem. Photobiol. Sci., 2002, 1, 592–596; (b) M. Norval and G. M. Woods, UV-induced immunosuppression and the efficacy of vaccination, Photochem. Photobiol. Sci., 2011, 10, 1267–1274.
R. Šmucler and E. Jatšová, Comparative Study of Aminolevulic Acid Photodynamic Therapy plus Pulsed Dye Laser versus Pulsed Dye Laser Alone in Treatment of Viral Warts, Photomed. Laser Surg., 2005, 23, 202–205; (b) A. B. Boehm, K. M. Yamahara, D. C. Love, B. M. Peterson, K. McNeill and K. L. Nelson, Covariation and Photoinactivation of Traditional and Novel Indicator Organisms and Human Viruses at a Sewage-Impacted Marine Beach, Environ. Sci. Technol., 2009, 43, 8046–8052; (c) M. B. Fisher, D. C. Love, R. Schuech and K. L. Nelson, Simulated Sunlight Action Spectra for Inactivation of MS2 and PRD1 Bacteriophages in Clear Water, Environ. Sci. Technol., 2011, 45, 9249–9255; (d) E. Grillo, P. Boixeda, A. Ballester, A. Miguel-Morrondo, T. Truchuelo and P. Jaén, Pulsed dye laser treatment for facial flat warts, Dermatol. Ther., 2014, 27, 31–35; (e) N. M. Reis and G. L. Puma, A novel microfluidic approach for extremely fast and efficient photochemical transformations in fluoropolymer microcapillary films, Chem. Commun., 2015, 51, 8414–8417; (f) Y. Ren, C. M. Crump, M. M. Mackley, G. L. Puma and N. M. Reis, Photo Inactivation of Virus Particles in Microfluidic Capillary Systems, Biotechnol. Bioeng., 2016, 113, 1481–1492; (g) R. M. Tomb, M. Maclean, J. E. Coia, E. Graham, M. McDonald, C. D. Atreya, S. J. MacGregor and J. G. Anderson, New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination, Food Environ. Virol., 2017, 9, 159–167; (h) I. Bodgan Allemann and J. Kaufman, Fractional photothermolysis – an update, Lasers Med. Sci., 2010, 25, 137–144; (i) A. Liu, R. L. Moy, E. Victor Ross, I. Hamzavi and D. M. Ozog, Pulsed dye laser and pulsed dye laser-mediated photodynamic therapy in the treatment of dermatologic disorders, Dermatol. Surg., 2012, 38, 351–366.
A. R. M. Chen-Collins, D. W. Dixon, A. N. Vzorov, L. G. Marzilli and R. W. Compans, Prevention of poxvirus infection by tetrapyrroles, BMC Infect. Dis., 2003, 3, 9; (b) Z. Li, M. Brecher, Y.-Q. Deng, J. Zhang, S. Sakamuru, B. Liu, R. Huang, C. A. Koetzner, C. A. Allen, S. A. Jones, H. Chen, N.-N. Zhang, M. Tian, F. Gao, Q. Lin, N. Banavali, J. Zhou, N. Boles, M. Xia, L. D. Kramer, C.-F. Qin and H. Li, Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction, Cell Res., 2017, 27, 1046–1064.
P. Hyckel, P. Schleier, A. Meerbach, A. Berndt, H. Kosmehl and P. Wutzler, The therapy of virus-associated epithelial tumors of the face and the lips in organ transplant recipients, Med. Microbiol. Immunol., 2003, 192, 171–176; (b) O. I. Trushina, E. G. Novikova, V. V. Sokolov, E. V. Filonenko, V. I. Chissov and G. N. Vorozhtsov, Photodynamic therapy of virus-associated precancer and early stages cancer of cervix uteri, Photodiagn. Photodyn. Ther., 2008, 5, 256–259; (c) H.-K. Koon, K.-W. Lo, K.-N. Leung, M. L. Lung, C. C.-K. Chang, R. N.-S. Wong, W.-N. Leung and N.-K. Mak, Photodynamic therapy-mediated modulation of inflammatory cytokine production by Epstein-Barr virus-infected nasopharyngeal carcinoma cells, Cell. Mol. Immunol., 2010, 7, 323–326.
P. Soergal, I. Staboulidou, C. Schippert and P. Hillemanns, Photodynamic diagnosis and therapy in gynecology, J. Environ. Pathol., Toxicol. Oncol., 2008, 27, 307–320; (b) P. Hillemanns, M. H. Einstein and O. E. Iversen, Topical hexaminolevulinate photodynamic therapy for the treatment of persistent human papilloma virus infections and cervical intraepithelial neoplasia, Expert Opin. Invest. Drugs, 2015, 24, 273–281; (c) K. W. Lai and M. G. Mercurio, Medical and surgical approaches to vulvar intraepithelial neoplasia, Dermatol. Ther., 2010, 25, 477–484; (d) Y. P. Istomin, T. P. Lapzevich, V. N. Chalau, S. V. Shliakhtsin and T. V. Trukhachova, Photodynamic therapy of cervical intraepithelial neoplasia grades II and III with Photolon®, Photodiagn. Photodyn. Ther., 2010, 7, 144–151; (e) C. J. M. Henquet, Anogenital malignancies and pre-malignancies, J. Eur. Acad. Dermatol. Venereol., 2011, 25, 885–895; (f) J. A. D. Simpson and J. H. Scholefield, Diagnosis and management of anal intraepithelial neoplasia and anal cancer, Br. Med. J., 2011, 343, 1004–1009; (g) J. Haedicke and T. Iftner, Human papillomaviruses and cancer, Radiother. Oncol., 2013, 108, 397–402; (h) F. X. Bosch, T. R. Broker, D. Forman, A.-B. Moscicki, M. L. Gillison, J. Doorbar, P. L. Stern, M. Stanley, M. Arbyn, M. Poljak, J. Cuzick, P. E. Castle, J. T. Schiller, L. E. Markowitz, W. A. Fisher, K. Canfell, L. A. Denny, E. L. Franco, M. Steben, M. A. Kane, M. Schiffman, C. J. L. M. Meijer, R. Sankaranarayanan, X. Castellsagué, J. J. Kim, M. Brotons, L. Alemany, G. Albero, M. Diaz, S. D. Sanjosé, A. Anttila, L. Banks, C. Bergeron, J. L. Belinson, J. Berkhof, I. G. Bravo, F. Bray, J. M. L. Brotherton, L. Bruni, A. Burchell, A. Chaturvedi, H. Chesson, M. Chevarie-Davis, H. Cubie, S. L. Deeks, C. de Martel, S. de Sanjosé, J. Dillner, M. H. Einstein, J. Ferlay, A. Fiander, S. Franceschi, S. M. Garland, A. R. Giuliano, M. T. Goodman, P. Gravitt, I. N. Hampson, I. Heard, T. Iftner, S. D. Isidean, C. Jensen, J. Jeronimo, W. Kinney, H. C. Kitchener, S. K. Kjaer, B. J. Kocjan, G. Koliopoulos, S. L. Kulasingam, C. J. Lacey, D. Scott LaMontagne, E. Lazcano-Ponce, A. T. Lorincz, J. Lortet-Tieulent, P. Naucler, G. Ogilvie, J. Palefsky, J. Peto, L. A. Pinto, M. Plummer, Y.-L. Qiao, W. Quint, G. Ronco, S. Schwartz, B. Serrano, J. Smith, P. J. F. Snijders, I. Soerjomataram, B. M. Steinberg, M. Stoler, A. Szarewski, C. Trimble, V. Tsu, P. van Damme, S. H. van der Burg, A. S. Vicari, J. Vignat, M. von Knebel Doeberitz, A. Vorsters, S. A. Wang and S. Wittet, Comprehensive Control of Human Papillomavirus Infections and Related Diseases, Vaccine, 2012, 30(Suppl. 5), F1–F202; (i) F. X. Bosch, T. R. Broker, D. Forman, A.-B. Moscicki, M. L. Gilson, J. Doorbar, P. L. Stern, M. Stanley, M. Arbyn, M. Poljak, J. Cuzick, P. E. Castle, J. T. Schiller, L. E. Markowitz, W. A. Fisher, K. Canfell, L. A. Denny, E. L. Franco, M. Steben, M. A. Kane, M. Schiffman, C. J. L. M. Meijer, R. Sankaranarayanan, X. Castellsagué, J. J. Kim, M. Brotons, L. Alemany, G. Albero, M. Diaz and S. D. Sanjosé, Comprehensive control of human papillomavirus infections and related diseases, Vaccine, 2013, 31(Suppl. 6), G1–G31.
P. L. Stern, S. H. van der Burg, I. N. Hampson, T. R. Broker, A. Fiander, C. J. Lacey, H. C. Kitchener and M. H. Einstein, Therapy of Human Papillomavirus-Related Disease, Vaccine, 2012, 30S, F71–F82; (b) R. W. Nims and M. Plavsic, Polyomavirus inactivation – a review, Biologicals, 2013, 41, 63–70; (c) R. Nims and M. Plavsic, Inactivation of calciviruses, Pharmaceuticals, 2013, 6, 358–392; (d) E. V. Filonenko and L. G. Serova, Photodynamic therapy in the clinical practice, Biomed. Photonics, 2016, 5, 26–37.
V. Mrázová, M. Kúdelová, M. Smolinská, E. Nováková, M. Šupolíková, M. Vrbová and F. Golais, Transformation of Cells by Photoinactivated Murine Gamma-Herpesvirus 68 during Nonproductive and Quiescent Infection, Intervirology, 2017, 60, 61–68.
H. C. Wolfsen and C. S. Ng, Cutaneous Consequences of Photodynamic Therapy, Cutis, 2002, 69, 140–142; (b) K.-C. Yoon, S.-K. Im and H.-Y. Park, Recurrent Herpes Simplex Keratitis After Verteporfin Photodynamic Therapy for Corneal Neovascularization, Cornea, 2010, 29, 465–467; (c) P. Lehmann, Nebenwirkungen der topischen photodynamischen Therapie, Hautarzt, 2007, 58, 597–603; (d) S. Nobbe, R. M. Trüeb, L. E. French and G. F. L. Hofbauer, Herpes simplex virus reactivation as a complication of photodynamic therapy, Photodermatol., Photoimmunol. Photomed., 2011, 27, 51–52; (e) K. Manno and J. L. Cohen, Temporal Association of Herpes Zoster Eruption Post- Aminolevulinic Acid Hydrochloride Photodynamic Therapy for Actinic Keratoses, J. Drugs Dermatol., 2017, 16, 817–818.
B.Ø Engesæter, S. Tveito, A. Bonsted, O. Engebraaten, K. Berg and G. M. Mælandsmo, Photochemical treatment with endosomally localized photosensitizers enhances the number of adenoviruses in the nucleus, J. Gene Med., 2006, 8, 707–718.
H. E. van Kan-Davelaar, J. C. M. van Hest, J. J. L. M. Cornelissen and M. S. T. Koay, Using viruses as nanomedicines, Br. J. Pharmacol., 2014, 171, 4001–4009; (b) L. Liang, W. Bi, W. Chen, Y. Lin and Y. Tian, Combination of MPPa-PDT and HSV1-TK/GCV gene therapy on prostate cancer, Lasers Med. Sci., 2018, 33, 227–232; (c) J.-K. Rhee, M. Baksh, C. Nycholat, J. C. Paulson, H. Kitagishi and M. G. Finn, Glycan-Targeted Virus-like Nanoparticles for Photodynamic Therapy, Biomacromolecules, 2012, 13, 2333–2338; (d) L. Bourré, F. Giuntini, I. M. Eggleston, C. A. Mosse, A. J. MacRobert and M. Wilson, Effective photoinactivation of Gram-positive and Gram-negative bacterial strains using an HIV-1 Tat peptide–porphyrin conjugate, Photochem. Photobiol. Sci., 2010, 9, 1613–1620; (e) J. P. Scaffidi, M. K. Gregas, B. Lauly, Y. Zhang and T. Vo-Dinh, Activity of Psoralen-Functionalized Nanoscintillators against Cancer Cells upon X-ray Excitation, ACS Nano, 2011, 5, 4679–4687; (f) N. Stephanopoulos, G. J. Tong, S. C. Hsiao and M. B. Francis, Dual-Surface Modified Virus Capsids for Targeted Delivery of Photodynamic Agents to Cancer Cells, ACS Nano, 2010, 4, 6014–6020; (g) E. K. Park, S.-M. Bae, S.-Y. Kwak, S. J. Lee, Y.-W. Kim, C.-H. Han, H.-J. Cho, K. T. Kim, Y.-J. Kim, H.-J. Kim and W. S. Ahn, Photodynamic therapy with recombinant adenovirus AdmIL-12 enhances enti-tumour therapy efficacy in human papillomavirus 16 (E6/E7) infected tumour model, Immunology, 2008, 124, 416–468.
A. M. Wen, M. J. Ryan, A. C. Yang, K. Breitenkamp, J. K. Pokorskid and N. F. Steinmetz, Photodynamic activity of viral nanoparticles conjugated with C60, Chem. Commun., 2012, 48, 9044–9046; (b) B. A. Cohen and M. Bergkvist, Targeted in vitro photodynamic therapy via aptamer-labeled, porphyrin-loaded virus capsids, J. Photochem. Photobiol., B, 2013, 121, 67–74; (c) B. A. Cohen, A. E. Kaloyeros and M. Bergkvist, MS2 bateriophage as a delivery vessel of porphyrins for photodynamic therapy, Proc. SPIE, 2011, 7886, 788614; (d) K. Takehara, H. Tazawa, N. Okada, Y. Hashimoto, S. Kikuchi, S. Kuroda, H. Kishimoto, Y. Shirakawa, N. Narii, H. Mizuguchi, Y. Urata, S. Kagawa and T. Fujiwara, Targeted Photodynamic Virotherapy Armed with a Genetically Encoded Photosensitizer, Mol. Cancer Ther., 2016, 15, 199–208; (e) K. L. Lee, B. L. Carpenter, A. M. Wen, R. A. Ghiladi and N. F. Steinmetz, High Aspect Ratio Nanotubes Formed by Tobacco Mosaic Virus for Delivery of Photodynamic Agents Targeting Melanoma, ACS Biomater. Sci. Eng., 2016, 2, 838–844.
S.-J. Tseng, K.-Y. Huang, I. M. Kempson, S.-H. Kao, M.-C. Liu, S.-C. Yang, Z.-X. Liao and P.-C. Yang, Remote Control of Light-Triggered Virotherapy, ACS Nano, 2016, 10, 10339–10346; (b) A. M. Wen, K. L. Lee, P. Cao, K. Pangilinan, B. L. Carpenter, P. Lam, F. A. Veliz, R. A. Ghiladi, R. C. Advincula and N. F. Steinmetz, Utilizing Viral Nanoparticle/Dendron Hybrid Conjugates in Photodynamic Therapy for Dual Delivery to Macrophages and Cancer Cells, Bioconjugate Chem., 2016, 27, 1227–1235; (c) M. Brasch, A. de la Escosura, Y. Ma, C. Uetrecht, A. J. R. Heck, T. Torres and J. J. L. M. Cornelissen, Encapsulation of phthalocyanine supramolecular stacks into virus-like particles, J. Am. Chem. Soc., 2011, 133, 6878–6881; (d) Y. Ma, R. J. M. Nolte and J. J. L. M. Cornelissen, Virus-based nanocarriers for drug delivery, Adv. Drug Delivery Rev., 2012, 64, 811–825; (e) S. K. Dixit, N. L. Goicochea, M.-C. Daniel, A. Murali, L. Bronstein, M. De, B. Stein, V. M. Rotello, C. C. Kao and B. Dragnea, Quantum Dot Encapsulation in Viral Capsids, Nano Lett., 2006, 6, 1993–1999.
M. Gil, M. Bieniasz, M. Seshadri, D. Fisher, M. J. Ciesielski, Y. Chen, R. K. Pandey and D. Kozbor, Photodynamic therapy augments the efficacy of oncolytic vaccinia virus against primary and metastatic tumours in mice, Br. J. Cancer, 2011, 105, 1512–1521.
D. M. Lin, B. Koskella and H. C. Lin, Phage therapy: An alternative to antibiotics in the age of multi-drug resistance, World J. Gastrointest. Pharmacol. Ther., 2017, 8, 162– 173; (b) A. Kiros, T. Gashaw and A. Teshale, Phage Therapy; A Review on the Biology and Therapeutic Application of Bacteriophage, ARC J. Animal Vet. Sci., 2016, 2, 15–25; (c) I. U. Haq, W. N. Chaudhry, M. N. Akhtar, S. Andleeb and I. Qadri, Bacteriophages and their implications on future biotechnology: a review, Virol. J., 2012, 9, 9, DOI: 10.1186/1743-422X-9-9.
O. J. Norum, P. K. Selbo, A. Weyergang, K. E. Giercksky and K. Berg, Photochemical internalization (PCI) in cancer therapy: from bench towards bedside medicine, J. Photochem. Photobiol., B, 2009, 96, 83–92.
Based on, for example, Scopus search for articles with “photodynamic and antiviral” or “photoinactivation and virus” or similar.
M. A. Pathak, Mechanisms of psoralen photosensitization reactions, Natl. Cancer Inst. Monogr., 1984, 66, 41–46.
M. Wainwright, The use of dyes in modern biomedicine, Biotech. Histochem., 2003, 78, 147–155.
R. Ackroyd, C. Kelty, N. Brown and M. Reed, The history of photodetection and photodynamic therapy, Photochem. Photobiol., 2001, 74, 656–669; (b) J. Moan and Q. Peng, An outline of the hundred-year history of PDT, Anticancer Res., 2003, 23, 3591–3600; (c) D. Kessel, Photodynamic therapy: from the beginning, Photodiagn. Photodyn. Ther., 2004, 1, 3–7; (d) A. Juzeniene, Q. Peng and J. Moan, Milestones in the development of photodynamic therapy and fluorescence diagnosis, Photochem. Photobiol. Sci., 2007, 6, 1234–1245; (e) H. Honigsmann, History of phototherapy in dermatology, Photochem. Photobiol. Sci., 2013, 12, 16–21; (f) R.-M. Szeimies, Geschichte der Photodynamischen Theraphie, Aktual. Dermatol., 2005, 31, 193–197.
K. A. Cengel, C. B. Simone II and E. Glatstein, PDT: What’s Past Is Prologue, Cancer Res., 2016, 76, 2497–2499.
N. R. Finsen, La Photothérapie, Georges Carré et C. Naud, Paris, 1899.
K. I. Møller, B. Konigshoj, P. A. Philipsen, V. O. Thomsen and H. C. Wulf, How Finsen’s light cured lupus vulgaris, Photodermatol., Photoimmunol. Photomed., 2005, 21, 118–124; (b) R. Roelandts, A new light on Niels Finsen, a century after his nobel prize, Photodermatol., Photoimmunol. Photomed., 2005, 21, 115–117.
J. Prime, Des accidents toxiques produit par l’éosine du sodium, Thesis, Joves et Boyer, Paris, 1900.
This and similar observations with related dyes actually preceed the classic use of haematoporphyrin as photosensitizer by Hausmann47a and Meyer-Betz’s famous self-administration thereof47b: W. Straub, Über chemische Vorgänge bei der Einwirkung von Licht auf fluoreszierende Substanzen (Eosin und Chinin) und die Bedeutung dieser Vorgänge für die Giftwirkung, Münch. Med. Wochenschr., 1904, 51, 1093–1096; (b) G. Dreyer, Lichtbehandlung nach Sensibilisierung, Dermatol. Z., 1903, 10, 578–580; (c) W. Hausmann, Über die sensibilisierende Wirkung tierischer Farbstoffe und ihre physiologische Bedeutung, Wien. Klin. Wochenschr., 1908, 21, 1527–1528.
W. Hausmann, Die sensibilisierende Wirkung des Hämatoporphyrins, Biochem. Z., 1911, 30, 276–316; (b) F. Meyer-Betz, Untersuchungen ueber die biologische (photodynamische) Wirkung des Haematoporphyrins und andere Derivate des Blut- und Gallenfarbstoffs, Dtsch. Arch. Klin. Med., 1913, 112, 476–503.
O. Raab, Über die Wirkung fluoreszierender Stoffe auf Infusorien, Z. Biol., 1900, 39, 524–546; (b) H. von Tappeiner, Über die Wirkung fluoreszierender Stoffe auf Infusorien nach Versuchen von O., Raab, Münch. Med. Wochenschr., 1900, 47, 5–7.
C. Ledoux-Lebards, Action de la lumiere sur la toxicite de l’éosine et de quelques autres substances, Ann. Inst. Pasteur, 1902, 16, 587–593.
H. von Tappeiner and A. Jodlbauer, Über Wirkung der photodynamischen (fluoreszierenden) Stoffe auf Protozoan und Enzyme, Dtsch. Arch. Klin. Med., 1904, 80, 427–487; (b) H. von Tappeiner and A. Jodlbauer, Die sensibilisierende Wirkung fluorescierender Substanzen; gesammelte Untersuchungen über die photodynamische Erscheinung, Vogel, Leipzig, 1907; (c) H. von Tappeiner, Die photodynamische Erscheinung (Sensibilisierung durch fluoreszierende Stoffe), Ergeb. Physiol., 1909, 8, 698– 941.
H. Jesionek and H. von Tappeiner, Therapeutische Versuche mit Fluoreszierenden Stoffen, Münch. Med. Wochenschr., 1903, 47, 2024–2044; (b) A. Jesionek, Lichtbiologie. Die experimentellen Grundlagen der modernen Lichtbehandlung, Vieweg, Braunschweig, 1910.
Early contributions reported, among others, on plant virus and Polyomyelitis and bacteriophage inactivation with methylene blue: E. W. Schultz and A. P. Krueger, Inactivation of Staphylococcus bacteriophage by methylene blue, Proc. Soc. Exp. Biol. Med., 1928, 26, 100–101; (b) H. H. Storey, The photodynamic action of methylene blue on the virus of a plant disease, Ann. Appl. Biol., 1934, 21, 588–589; (c) J. M. Birkeland, Photodynamic action of methylene blue on plant viruses, Science, 1934, 80, 357– 358; (d) L. A. Rosenblum, B. Hoskwith and S. D. Kramer, Photodynamic Action of Methylene Blue on Poliomyelitis Virus, Exp. Biol. Med., 1937, 37, 166–168. See also ref. 7a, 9 and 40.
B. L. Aronoff, Lasers: Reflections on their evolution, J. Surg. Oncol., 1997, 64, 84–92.
A typical example is a report on photodynamic inactivation of arboviruses and VSV by compounds extracted from algae: T. Fukada, M. Hoshino, H. Endo, M. Mutai and M. Shirota, Photodynamic Antiviral Substance Extracted from Chlorella Cells, Appl. Microbiol., 1968, 16, 1809–1810.
F. Rapp, J. L. H. Li and M. Jerkofsky, Transformation of mammalian-cells by DNA-containing viruses following photodynamic inactivation, Virology, 1973, 55, 339–346; (b) L. Weinstein and T. W. Chang, Chemotherapy of viral infections, N. Engl. J. Med., 1973, 289, 725–730; (c) for an account of developments of this story see: J. L. Melnick and C. Wallis, Photodynamic inactivation of herpes simplex virus: a status report, Ann. N. Y. Acad. Sci., 1977, 284, 171–181.
R. A. Floyd, J. Edward Schneider Jr. and D. P. Dittmer, Methylene blue photoinactivation of RNA viruses, Antiviral Res., 2004, 61, 141–151.
L. B. Josefsen and R. W. Boyle, Photodynamic Therapy and the Development of Metal-Based Photosensitisers, Met.-Based Drugs, 2008, 276109.
A. P. Castano, T. N. Demidova and M. R. Hamblin, Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization, Photodiagn. Photodyn. Ther., 2004, 1, 279–293.
R. Bonnett, Chemical Aspects of Photodynamic Therapy, Gordon and Breach Science Publishers, Amsterdam, The Netherlands, 2000.
J.-A. See, S. Shumack, D. F. Murrell, D. M. Rubel, P. Fernández-Peñas, R. Salmon, D. Hewitt, P. Foley and L. Spelman, Consensus recommendations on the use of daylight photodynamic therapy with methyl aminolevulinate cream for actinic keratoses in Australia, Australas. J. Dermatol., 2016, 57, 167–174; (b) S. Ibbotson, R. Stones, J. Bowling, S. Campbell, S. Kownacki, M. Sivaramakrishnan, R. Valentine and C. A. Morton, A consensus on the use of daylight photodynamic therapy in the UK, J. Dermatol. Treat., 2017, 28, 360–367.
I. Fridovich, Superoxide Anion Radical (O2.−), Superoxide Dismutases, and Related Matters, J. Biol. Chem., 1997, 272, 18515–18517.
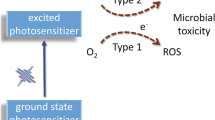
M. S. Baptista, J. Cadet, P. Di Mascio, A. A. Ghogare, A. Greer, M. R. Hamblin, C. Lorente, S. C. Nunez, M. S. Ribeiro, A. H. Thomas, M. Vignoni and T. M. Yoshimura, Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways, Photochem. Photobiol., 2017, 93, 912–919.
M. C. DeRosa and R. J. Crutchley, Photosensitized singlet oxygen and its applications, Coord. Chem. Rev., 2002, 233–234, 351–371.
H. Abrahamse and M. R. Hamblin, New photosensitizers for photodynamic therapy, Biochem. J., 2016, 473, 347– 364.
J. R. Kanofsky, Measurement of Singlet-Oxygen In Vivo: Progress and Pitfalls, Photochem. Photobiol., 2011, 87, 14–17; (b) M. T. Jarvi, M. J. Niedre, M. S. Patterson and B. C. Wilson, The Influence of Oxygen Depletion and Photosensitizer Triplet-state Dynamics During Photodynamic Therapy on Accurate Singlet Oxygen Luminescence Monitoring and Analysis of Treatment Dose Response, Photochem. Photobiol., 2011, 87, 223–234; (c) P. R. Ogilby, Singlet oxygen: there is indeed something new under the sun, Chem. Soc. Rev., 2010, 39, 3181–3209; (d) M. T. Jarvi, M. J. Niedre, M. S. Patterson and B. C. Wilson, Singlet Oxygen Luminescence Dosimetry (SOLD) for Photodynamic Therapy: Current Status, Challenges and Future Prospects, Photochem. Photobiol., 2006, 82, 1198–1210; (e) L. D. Via and S. M. Magno, Photochemotherapy in the Treatment of Cancer, Curr. Med. Chem., 2001, 8, 1405–1418; (f) D. B. Min and J. M. Boff, Chemistry and Reaction of Singlet Oxygen in Foods, Compr. Rev. Food Sci. Food Saf., 2002, 1, 58–72.
B. W. Henderson, S. O. Gollnick, J. W. Snyder, T. M. Busch, P. C. Kousis, R. T. Cheney and J. Morgan, Choice of Oxygen-Conserving Treatment Regimen Determines the Inflammatory Response and Outcome of Photodynamic Therapy of Tumors, Cancer Res., 2004, 64, 2120–2126.
J. H. Woodhams, A. J. MacRobert and S. G. Bown, The role of oxygen monitoring during photodynamic therapy and its potential for treatment dosimetry, Photochem. Photobiol. Sci., 2007, 6, 1246–1256.
S. Hackbarth, J. Schlothauer, A. Preuß and B. Röder, New insights to primary photodynamic effects – Singlet oxygen kinetics in living cells, J. Photochem. Photobiol., B, 2010, 98, 173–179; (b) A. Looft, M. Pfitzner, A. Preuß and B. Röder, In vivo singlet molecular oxygen measurements: Sensitive to changes in oxygen saturation during PDT, Photodiagn. Photodyn. Ther., 2018, 23, 325–330.
S. Mallidi, S. Anbil, A.-L. Bulin, G. Obaid, M. Ichikawa and T. Hasan, Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy, Theranostics, 2016, 6, 2458–2487; (b) A. Müller, A. Preuß and B. Röder, Photodynamic inactivation of Escherichia coli - Correlation of singlet oxygen kinetics and phototoxicity, J. Photochem. Photobiol., B, 2018, 178, 219–227.
L. Brancaleon and H. Moseley, Laser and Non-laser Light Sources for Photodynamic Therapy, Lasers Med. Sci., 2002, 17, 173–186; (b) T. S. Mang, Lasers and light sources for PDT: past, present and future, Photodiagn. Photodyn. Ther., 2004, 1, 43–48.
S. J. Wagner, J. R. Storry, D. A. Mallory, R. R. Stromberg, L. E. Benade and L. I. Friedman, Red cell alterations associated with virucidal methylene blue phototreatment, Transfusion, 1993, 33, 30–36.
H. Mohr, B. Bachmann, A. Klein-Struckmeier and B. Lambrecht, Virus inactivation of blood products by phenothiazine dyes and light, Photochem. Photobiol., 1997, 65, 441–445.
H. Abe, K. Ikebuchi, S. J. Wagner, M. Kuwabara, N. Kamo and S. Sekiguchi, Potential involvement of both type I and type II mechanisms in M13 virus inactivation by methylene blue photosensitization, Photochem. Photobiol., 1997, 66, 204–208.
Q. Huang, W.-L. Fu, B. Chen, J.-F. Huang, X. Zhang and Q. Xue, Inactivation of dengue virus by methylene blue/ narrow bandwidth light system, J. Photochem. Photobiol., B, 2004, 77, 39–43.
L. Costa, C. M. B. Carvalho, M. A. F. Faustino, M. G. P. M. S. Neves, J. P. C. Tomé, A. C. Tomé, J. A. S. Cavaleiro, A. Cunha and A. Almeida, Sewage bacteriophage inactivation by cationic porphyrins: influence of light parameters, Photochem. Photobiol. Sci., 2010, 9, 1126–1133.
M. A. Latief, T. Chikama, M. Shibasaki, T. Sasaki, J.-A. Ko, Y. Kiuchi, T. Sakaguchi and A. Obana, Antimicrobial action from a novel porphyrin derivative in photodynamic antimicrobial chemotherapy in vitro, Lasers Med. Sci., 2015, 30, 383–387; (b) M. A. Latief, T. Chikama, J.-A. Ko, Y. Kiuchi, T. Sakaguchi and A. Obana, Inactivation of acyclovir-sensitive and -resistant strains of herpes simplex virus type 1 in vitro by photodynamic antimicrobial chemotherapy, Mol. Vision, 2015, 21, 532–537.
A. Wiehe, E. J. Simonenko, M. O. Senge and B. Röder, Hydrophilicity vs hydrophobicity—varying the amphiphilic structure of porphyrins related to the photosensitizer m-THPC, J. Porphyrins Phthalocyanines, 2001, 5, 758–761; (b) S. Ben-Dror, I. Bronshtein, A. Wiehe, B. Röder, M. O. Senge and B. Ehrenberg, On the correlation between hydrophobicity, liposome binding and cellular uptake of porphyrin sensitizers, Photochem. Photobiol., 2006, 82, 695–701.
I. Laville, T. Figueiredo, B. Loock, S. Pigaglio, P. Maillard, D. S. Grierson, D. Carrez, A. Croisy and J. Blais, Synthesis, Cellular Internalization and Photodynamic Activity of Gluco-conjugated Derivatives of Tri and Tetra(meta-hydroxyphenyl)chlorins, Bioorg. Med. Chem., 2003, 11, 1643–1652; (b) R. Daly, G. Vaz, A. M. Davies, M. O. Senge and E. M. Scanlan, Synthesis and Biological Evaluation of a Library of Glycoporphyrin Compounds, Chem. – Eur. J., 2012, 18, 14671–14679; (c) B. Chauvin, B. I. Iorga, P. Chaminade, J.-L. Paul, P. Maillard, P. Prognon and A. Kasselouri, Plasma distribution of tetraphenylporphyrin derivatives relevant for Photodynamic Therapy: Importance and limits of hydrophobicity, Eur. J. Pharm. Biopharm., 2013, 83, 244–252.
C. Simões, M. C. Gomes, M. G. P. M. S. Neves, Â. Cunha, J. P. C. Tomé, A. C. Tomé, J. A. S. Cavaleiro, A. Almeida and M. A. F. Faustino, Photodynamic inactivation of Escherichia coli with cationic meso-tetraarylporphyrins – The charge number and charge distribution effects, Catal. Today, 2016, 266, 197–204.
A. G. Cobián Güemes, M. Youle, V. A. Cantú, B. Felts, J. Nulton and F. Rohwer, Viruses as Winners in the Game of Life, Annu. Rev. Virol., 2016, 3, 197–214.
A. K. Wiethoelter, D. Beltrán-Alcrudo, R. Kock and S. M. Mor, Global trends in infectious diseases at the wildlife–livestock interface, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 9662–9667.
M. Lorizate and H.-G. Kräusslich, Role of Lipids in Virus Replication, Cold Spring Harbor Perspect. Biol., 2011, 3, a004820; (b) C. F. O. Rodrigues Melo, J. Delafiori, M. Z. Dabaja, D. Noin de Oliveira, T. M. Guerreiro, T. E. Colombo, M. L. Nogueira, J. L. Proenca-Modena and R. Ramos Catharino, The role of lipids in the inception, maintenance and complications of dengue virus infection, Sci. Rep., 2018, 8, 11826, DOI: 10.1038/s41598-018-30385-x.
F. Käsermann and C. Kempf, Photodynamic inactivation of enveloped viruses by buckminsterfullerene, Antiviral Res., 1997, 34, 65–70.
S. Rywkin, E. Ben-Hur, Z. Malik, A. M. Prince, Y.-S. Li, M. E. Kenney, N. L. Oleinick and B. Horowitz, New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates, Photochem. Photobiol., 1994, 60, 165–170.
T. Cajka and O. Fiehn, Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry, Trends Anal. Chem., 2014, 61, 192–206.
A. W. Girotti, Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms, J. Photochem. Photobiol., B, 2001, 63, 103–113.
Z. Smetana, E. Ben-Hur, E. Mendelson, S. Salzberg, P. Wagner and Z. Malik, Herpes simplex virus proteins are damaged following photodynamic inactivation with phthalocyanines, J. Photochem. Photobiol., B, 1998, 44, 77–83.
S. Rywkin, L. Lenny, J. Goldstein, N. E. Geactinov, H. Margolis-Nunno and B. Horowitz, Importance of Type I and Type II mechanisms in the photodynamic inactivation of viruses in blood with aluminum phthalocyanine derivatives, Photochem. Photobiol., 1992, 56, 463–469.
L. Costa, M. A. F. Faustino, J. P. C. Tomé, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, Â. Cunha and A. Almeida, Involvement of type I and type II mechanisms on the photoinactivation of non-enveloped DNA and RNA bacteriophages, J. Photochem. Photobiol., B, 2013, 120, 10–16.
A. R. Badireddy, E. M. Hotze, S. Chellam, P. J. J. Alvarez and M. R. Wiesner, Inactivation of bacteriophages via photosensitization of fullerol nanoparticles, Environ. Sci. Technol., 2007, 41, 6627–6632.
F. Gábor, J. Szolnoki, K. Tóth, A. Fekete, P. Maillard and G. Csík, Photoinduced inactivation of T7 phage sensitized by symmetrically and asymmetrically substituted tetraphenyl porphyrin: comparison of efficiency and mechanism of action, Photochem. Photobiol., 2001, 73, 304–311; (b) M. Egyeki, G. Turóczy, Z. Majer, K. Tóth, A. Fekete, P. Maillard and G. Csík, Photosensitized inactivation of T7 phage as surrogate of non-enveloped DNA viruses: Efficiency and mechanism of action, Biochim. Biophys. Acta, 2003, 1624, 115–124.
E. M. Hotze, A. R. Badireddy, S. Chellam and M. R. Wiesner, Mechanisms of Bacteriophage Inactivation via Singlet Oxygen Generation in UV Illuminated Fullerol Suspensions, Environ. Sci. Technol., 2009, 43, 6639–6645.
R. B. Greenberg, M. Alberti, J. E. Hearst, M. A. Chua and W. A. Saffran, Recombinational and Mutagenic Repair of Psoralen Interstrand Cross-links in Saccharomyces cerevisiae, J. Biol. Chem., 2001, 276, 31551–31560; (b) J. M. Mundt, L. Rouse, J. Van den Bossche and R. P. Goodrich, Chemical and Biological Mechanisms of Pathogen Reduction Technologies, Photochem. Photobiol., 2014, 90, 957–964.
W.-Y. Li, J.-G. Xu and X.-W. He, Characterization of the Binding of Methylene Blue to DNA by Spectroscopic Methods, Anal. Lett., 2000, 33, 2453–2464; (b) M. Hossain and G. S. Kumar, DNA intercalation of methylene blue and quinacrine: new insights into base and sequence specificity from structural and thermodynamic studies with polynucleotides, Mol. BioSyst., 2009, 5, 1311–1322; (c) P. O. Vardevanyan, A. P. Antonyan, M. A. Parsadanyan, M. A. Shahinyan and L. A. Hambardzumyan, Mechanisms for binding between methylene blue and DNA, J. Appl. Spectrosc., 2013, 80, 595–599.
B. Zhang, L. Zheng, Y. Huang, Q. Mo, X. Wang and K. Qian, Detection of Nucleic Acid Lesions During Photochemical Inactivation of RNA Viruses by Treatment with Methylene Blue and Light Using Real-time PCR, Photochem. Photobiol., 2011, 87, 365–369.
I. Banerjee, M. P. Douaisi, D. Mondal and R. S. Kane, Light-activated nanotube–porphyrin conjugates as effective antiviral agents, Nanotechnology, 2012, 23, 105101, DOI: 10.1088/0957-4484/23/10/105101.
L. S. Kucera, J. P. Gusdon, I. Edwards and G. Herbst, Oncogenic transformation of rat embryo fibroblasts with photoinactivated herpes simplex virus: rapid in vitro cloning of transformed cells, J. Gen. Virol., 1977, 35, 473– 485.
H. Majiya, O. O. Adeyemib, N. J. Stonehouse and P. Millner, Photodynamic inactivation of bacteriophage MS2: The A-protein is the target of virus inactivation, J. Photochem. Photobiol., B, 2018, 178, 404–411.
A. W. Jensen, S. R. Wilson and D. I. Schuster, Biological Applications of Fullerenes, Bioorg. Med. Chem., 1996, 4, 767–779; (b) Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes, ed. F. Cataldo and T. Da Ros, Springer, Berlin, 2008; (c) P. Anilkumar, F. Lu, L. Cao, P. G. Luo, J.-H. Liu, S. Sahu, K. N. Tackett II, Y. Wang and Y. P. Sun, Fullerenes for Applications in biology and Medicine, Curr. Med. Chem., 2011, 18, 2045–2059.
British Society for Immunology, J. Goulding, Virus Replication, retrieved July 03, 2019 from https://www.immunology.org/public-information/bitesized-immunology/patógenos-y-enfermedades/virus-replication.
K. Morikawa, G. Suda and N. Sakamoto, Viral life cycle of hepatitis B virus: Host factors and druggable targets, Hepatol. Res., 2016, 46, 871–877.
E. De Clercq and G. Li, Approved Antiviral Drugs over the Past 50 Years, Clin. Microbiol. Rev., 2016, 29, 695–747.
J. Lenard and R. Vanderoef, Photoinactivation of influenza virus fusion and infectivity by rose bengal, Photochem. Photobiol., 1993, 58, 527–531; (b) J. Lenard, A. Rabson and R. Vanderoef, Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: Inhibition of fusion and syncytia formation, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 158–162.
A. L.-A. Monjo, E. S. Pringle, M. Thornbury, B. A. Duguay, S. M. A. Monro, M. Hetu, D. Knight, C. G. Cameron, S. A. McFarland and C. McCormick, Photodynamic activation of herpes simplex viruses, Viruses, 2018, 10, 532.
J. M. Belanger, Y. Raviv, M. Viard, J. M. de la Cruz, K. Nagashima and R. Blumenthal, Characterization of the effects of aryl-azido compounds and UVA irradiation on the viral proteins and infectivity of human immunodeficiency virus type 1, Photochem. Photobiol., 2010, 86, 1099– 1108; (b) J. M. Belanger, Y. Raviv, M. Viard, M. J. de la Cruz, K. Nagashima and R. Blumenthal, Effects of UVA irradiation, aryl azides, and reactive oxygen species on the orthogonal inactivation of the human immunodeficiency virus (HIV-1), Virology, 2011, 417, 221–228.
K. L. Warfield, D. L. Swenson, G. G. Olinger, W. V. Kalina, M. Viard, M. Aitichou, X. Chi, S. Ibrahim, R. Blumenthal, Y. Raviv, S. Bavari and M. J. Aman, Ebola Virus Inactivation with Preservation of Antigenic and Structural Integrity by a Photoinducible Alkylating Agent, J. Infect. Dis., 2007, 196, S276–S283.
Y. Raviv, R. Blumenthal, S. M. Tompkins, J. Humberd, R. J. Hogan and M. Viard, Hydrophobic Inactivation of Influenza Viruses Confers Preservation of Viral Structure with Enhanced Immunogenicity, J. Virol., 2008, 82, 4612– 4619.
J. Dairou, C. Vever-Bizet and D. Brault, Interaction of sulfonated anionic porphyrins with HIV glycoprotein gp120: photodamages revealed by inhibition of antibody binding to V3 and C5 domains, Antiviral Res., 2004, 61, 37–47.
S. Colby-Germinario, A. Rios, J. Quesada, D. Anderson, A. L. Goldstein, T. Fossum and M. A. Wainberg, HIV inactivation by cross-linking of photo-labeled anti-retroviral compounds with HIV reverse transcriptase, Vaccine, 2009, 27, 6137–6142.
F. Vigant, J. Lee, A. Hollmann, L. B. Tanner, Z. A. Ataman, T. Yun, G. Shui, H. C. Aguilar, D. Zhang, D. Meriwether, G. Roman-Sosa, L. R. Robinson, T. L. Juelich, H. Buczkowski, S. Chou, M. A. R. B. Castanho, M. C. Wolf, J. K. Smith, A. Banyard, M. Kielian, S. Reddy, M. R. Wenk, M. Selke, N. C. Santos, A. N. Freiberg, M. E. Jung and B. Lee, A Mechanistic Paradigm for Broad-Spectrum Antivirals that Target Virus-Cell Fusion, PLOS Pathog., 2013, 9, e1003297.
F. Vigant, A. Hollmann, J. Lee, N. C. Santos, M. E. Jung and B. Lee, The Rigid Amphipathic Fusion Inhibitor dUY11 Acts through Photosensitization of Viruses, J. Virol., 2014, 88, 1849–1853.
W. Thongthai and K. Weninger, Photoinactivation of Sindbis Virus Infectivity Without Inhibition of Membrane Fusion, Photochem. Photobiol., 2009, 85, 801–806.
F. Ayala, E. Grimaldi, B. Perfetto, M. Donnarumma, A. De Filippis, G. Donnarumma and M. A. Tufano, 5-Aminolaevulinic acid and photodynamic therapy reduce HSV-1 replication in HaCat cells through an apoptosis-independent mechanism, Photodermatol., Photoimmunol. Photomed., 2008, 24, 237–243.
A. C. E. Moor, A. E. Wagenaars-van Gompel, A. Brand, T. M. A. R. Dubbelman and J. VanSteveninck, Primary targets for photoinactivation of Vesicular stomatitis virus by AIPcS4 or Pc4 and Red Light, Photochem. Photobiol., 1997, 65, 465–470 see also: B. R. Munson and R. J. Fiel, Hematoporphyrin-sensitized photodynamic inactivation of viral RNA-dependent DNA polymerase, Res. Commun. Chem. Pathol. Pharmacol., 1977, 16, 175–178.
G. Sofer, D. C. Lister and J. A. Boose, Virus Inactivation in the 1990s —and into the 21st Century. Part 6, Inactivation Methods Grouped by Virus, BioPharm Int., 2003, 16, 42–68.
E. Postnikova, Y. Cong, L. E. DeWald, J. Dyall, S. Yu, B. J. Har, H. Zhou, R. Gross, J. Logue, Y. Cai, N. Deiuliis, J. Michelotti, A. N. Honko, R. S. Bennett, M. R. Holbrook, G. G. Olinger, L. E. Hensley and P. B. Jahrling, Testing therapeutics in cell-based assays: Factors that influence the apparent potency of drugs, PLoS One, 2018, 13, e0194880.
J. M. Hu and G. D. Hsiung, Evaluation of new antiviral agents: I. In vitro perspectives, Antiviral Res., 1989, 11, 217–232.
M. Rumlová and T. Ruml, In vitro methods for testing antiviral drugs, Biotechnol Adv., 2018, 36, 557–576.
Z. S. Silva Jr., S. K. Bussadori, K. P. S. Fernandes, Y.-Y. Huang and M. R. Hamblin, Animal models for photodynamic therapy (PDT), Biosci. Rep., 2015, 35, e00265.
T. Da Ros and M. Prato, Medicinal chemistry with fullerenes and fullerene derivatives, Chem. Commun., 1999, 663–669.
M. A. Filatov and M. O. Senge, Molecular devices based on reversible singlet oxygen binding in optical and photomedical applications, Mol. Syst. Des. Eng., 2016, 1, 258–272; (b) S. Callaghan and M. O. Senge, The Good, the Bad, and the Ugly – Controlling Singlet Oxygen through Design of Photosensitizers and Delivery Systems for Photodynamic Therapy, Photochem. Photobiol. Sci., 2018, 17, 1490–1514.
H. P. T. Ammon and M. A. Wahl, Pharmacology of Curcuma longa, Planta Med., 1991, 57, 1–7; (b) S. Z. Moghadamtousi, H. A. Kadir, P. Hassandarvish, H. Tajik, S. Abubakar and K. Zandi, A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin, BioMed Res. Int., 2014, 2014, 186864.
T. A. Dahl, P. Bilski, K. J. Reszka and C. F. Chignell, Photocytotoxicity of curcumin, Photochem. Photobiol., 1994, 58, 290–204.
V. Ravindranath and N. Chandrasekhara, Metabolism of curcumin – studies with [3H]curcumin, Toxicology, 1981, 22, 337–344.
R. K. Singh, D. Rai, D. Yadav, A. Bhargava, J. Balzarini and E. De Clercq, Synthesis, antibacterial and antiviral properties of curcumin bioconjugates bearing dipeptide, fatty acids and folic acid, Eur. J. Med. Chem., 2010, 45, 1078– 1086.
C. S. Divya and M. R. Pillai, Antitumor Action of Curcumin in Human Papillomavirus Associated Cells Involves Downregulation of Viral Oncogenes, Prevention of NFΚB and AP-1 Translocation, and Modulation of Apoptosis, Mol. Carcinog., 2006, 45, 320–332; (b) B. K. Prusty and D. C. Das, Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin, Int. J. Cancer, 2005, 113, 951–960.
D.-Y. Chen, J.-H. Shien, L. Tiley, S.-S. Chiou, S.-Y. Wang, T.-J. Chang, Y.-J. Lee, K.-W. Chan and W.-L. Hsu, Curcumin inhibits influenza virus infection and haemagglutination activity, Food Chem., 2010, 119, 1346–1351.
J. Wu, W. Hou, B. Cao, T. Zuo, C. Xue, A. W. Leung, C. Xu and Q.-J. Tang, Virucidal efficacy of treatment with photodynamically activated curcumin on murine norovirus bio-accumulated in oysters, Photodiagn. Photodyn. Ther., 2015, 12, 385–392; (b) W. Randazzo, R. Aznar and G. Sánchez, Curcumin-Mediated Photodynamic Inactivation of Norovirus Surrogates, Food Environ. Virol., 2016, 8, 244–250.
C. J. Li, L. J. Zhang, B. J. Dezube, C. S. Crumpacker and A. B. Pardee, Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 1839–1842.
A. Mazumder, K. Raghavan, J. Weinstein, K. W. Korn and Y. Pommier, Inhibition of Human Immunodeficiency Virus Type-1 Integrase by Curcumin, Biochem. Pharmacol., 1995, 49, 1165–1170.
Z. Sui, R. Salto, J. Li, C. Craik and P. R. Ortiz de Montellano, Inhibition of HIV-1 and HIV-2 Proteases by Curcumin and Curcumin Boron Complexes, Bioorg. Med. Chem., 1993, 1, 415–422.
S. Barthelemy, L. Vergnes, M. Moynier, D. Guyot, S. Labidalle and E. Bahraoui, Curcumin and curcumin derivatives inhibit Tat-mediated transactivation of type 1 human immunodeficiency virus long terminal repeat, Res. Virol., 1998, 149, 43–52; (b) K. Balasubramanyam, R. A. Varier, M. Altaf, V. Swaminathan, N. B. Siddappa, U. Range and T. K. Kundu, Curcumin, a Novel p300/ CREB-binding Protein-specific Inhibitor of Acetyltransferase, Represses the Acetylation of Histone/ Nonhistone Proteins and Histone Acetyltransferase-dependent Chromatin Transcription, J. Biol. Chem., 2004, 279, 51163–51171.
S. B. Kutluay, J. Doroghazi, M. E. Roemer and S. J. Triezenberg, Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity, Virology, 2008, 373, 239–247; (b) K. Zandi, E. Ramedani, K. Mohammadi, S. Tajbakhsh, I. Deilami, Z. Rastian, M. Foulavand, F. Yousefi and F. Farshadpar, Evaluation of Antiviral Activities of Curcumin Derivatives against HSV-1 in Vero Cell Line, Nat. Prod. Commun., 2010, 5, 1935–1938; (c) K. Z. Bourne, N. Bourne, S. F. Reising and L. R. Stanberry, Plant products as topical microbicide candidates: assessment of in vitro and in vivo activity against herpes simplex virus type 2, Antiviral Res., 1999, 42, 219– 226.
H. J. Kim, H. S. Yoo, J. C. Kim, C. S. Park, M. S. Choi, M. Kim, H. Choi, J. S. Min, Y. S. Kim, S. W. Yoon and J. K. Ahn, Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication, J. Ethnopharmacol., 2009, 124, 189–196; (b) K. Kim, K. H. Kim, H. Y. Kim, H. K. Cho, N. Sakamoto and J.-H. Cheong, Curcumin inhibits hepatits C virus replication via suppressing the Akt-SREBP-1 pathway, FEBS Lett., 2010, 584, 707–712; (c) X. Si, Y. Wang, J. Wong, J. Zhang, B. M. McManus and H. Luo, Dysregulation of the Ubiquitin-Proteasome System by Curcumin Suppresses Coxsackievirus B3 Replication, J. Virol., 2007, 81, 3142–3150; (d) K. Dutta, D. Ghosh and A. Basu, Curcumin Protects Neuronal Cells from Japanese Encephalitis Virus-Mediated Cell Death and also Inhibits Infective Viral Particle Formation by Dysregulation of Ubiquitin-Proteasome System, J. Neuroimmune Pharmacol., 2009, 4, 328–337.
C. A. Mulrooey, E. M. O’Brien, B. J. Morgan and M. C. Kozlowski, Perylenequinones: Isolation, Synthesis, and Biological Activity, Eur. J. Org. Chem., 2012, 3887– 3904.
A. Karioti and A. R. Bilia, Hypericins as Potential Leads for New Therapeutics, Int. J. Mol. Sci., 2010, 11, 562–594; (b) Z. Diwu, Novel therapeutic and diagnostic applications of hypocrellins and hypericins, Photochem. Photobiol., 1995, 61, 529–539.
M. Dostalek and A.-K. Stark, St John’s Wort (Hypericum Perforatum L.), in Metabolism of Drugs and Other Xenobiotics, ed. P. Anzenbacher and U. M. Zanger, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012, pp. 583–610.
H. Brockmann, F. Pohl, K. Maier and M. N. Haschad, Über das Hypericin, den photodynamischen Wirkstoff des Johanniskrautes (Hypericum perforatum), Justus Liebigs Ann. Chem., 1942, 553, 1–52.
G. Di Carlo, F. Borrelli, E. Ernst and A. A. Izzo, St. John’s wort. Prozac from the plant kingdom, Trends Pharmacol. Sci., 2001, 2, 292–297; (b) J. Barnes, L. A. Anderson and J. D. Phillipson, St John’s wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties, J. Pharm. Pharmacol., 2001, 53, 583–600.
S. Chrubasik-Hausmann, J. Vlachojannis and A. J. McLachlan, Understanding drug interactions with St John’s wort (Hypericum perforatum L.): impact of hyperforin content, J. Pharm. Pharmacol., 2019, 71, 129– 138; (b) C. M. Schempp, K. A. Müller, B. Winghofer, E. Schöpf and J. C. Simon, Johanniskraut (hypericum perforatum L.). Eine Pflanze mit Relevanz für die Dermatologie, Hautarzt, 2002, 53, 316–321.
M. Waser and H. Falk, Towards Second Generation Hypericin Based Photosensitizers for Photodynamic Therapy, Curr. Org. Chem., 2007, 11, 547–558; (b) M. Waser and H. Falk, Progress in the Chemistry of Second Generation Hypericin Based Photosensitizers, Curr. Org. Chem., 2011, 15, 3894–3907.
Z. Jendželovská, R. Jendželovský, B. Kuchárová and P. Fedoročko, Hypericin in the Light and in the Dark: Two Sides of the Same Coin, Front. Plant Sci., 2016, 7, 560, DOI: 10.3389/fpls.2016.00560.
V. Huntosova and K. Stroffekova, Hypericin in the Dark: Foe or Ally in Photodynamic Therapy?, Cancers, 2016, 8, 93, DOI: 10.3390/cancers8100093.
J. Berlanda, T. Kiesslich, V. Engelhardt, B. Krammer and K. Plaetzer, Comparative in vitro study on the characteristics of different photosensitizers employed in PDT, J. Photochem. Photobiol., B., 2010, 100, 173–180; (b) E. Besic Gyenge, P. Forny, D. Lüscher, A. Laass, H. Walt and C. Maake, Effects of hypericin and a chlorin based photosensitizer alone or in combination in squamous cell carcinoma cells in the dark, Photodiagn. Photodyn. Ther., 2012, 9, 321–331.
I. Lopez-Bazzocchi, J. B. Hudson and G. H. N. Towers, Antiviral activity of the photoactive plant pigment hypericin, Photochem. Photobiol., 1991, 54, 95–98; (b) S. Carpenter and G. A. Kraus, Photosensitization is required for inactivation of equine infectious anemia virus by hypericin, Photochem. Photobiol., 1991, 53, 169– 174.
A. Kubin, F. Wierrani, U. Burner, G. Alth and W. Grünberger, Hypericin - The Facts About a Controversial Agent, Curr. Pharm. Des., 2005, 11, 233–253.
D. Meruelo, Y. Mazur, D. Lavie, A. M. Prince, D. Pascual, L. Liebes and B. Levin, Hypericin as an inactivator of intectious viruses in blood components, Transfusion, 1995, 35, 392–400; (b) A. M. Prince, D. Pascual, D. Meruelo, L. Liebes, Y. Mazur, E. Dubovi, M. Mandel and G. Lavie, Strategies for evaluation of enveloped virus inactivation in red cell concentrates using hypericin, Photochem. Photobiol., 2000, 71, 188–195.
R. M. Gulick, V. McAuliffe, J. Holden-Wiltse, C. Crumpacker, L. Liebes, D. S. Stein, P. Meehan, S. Hussey, J. Forcht and F. T. Valentine, Phase I studies of hypericin, the active compound in St. John’s Wort, as an antiretroviral agent in HIV-infected adults. AIDS Clinical Trials Group Protocols 150 and 258, Ann. Intern. Med., 1999, 130, 510–514.
J. M. Jacobson, L. Feinman, L. Liebes, N. Ostrow, V. Koslowski, A. Tobia, B. E. Cabana, D.-H. Lee, J. Spritzler and A. M. Prince, Pharmacokinetics, Safety, and Antiviral Effects of Hypericin, a Derivative of St. John’s Wort Plant, in Patients with Chronic Hepatitis C Virus Infection, Antimicrob. Agents Chemother., 2001, 45, 517–524.
J. B. Hudson, L. Harris and G. H. N. Towers, The importance of light in the anti-HIV effect of hypericin, Antiviral Res., 1993, 20, 173–178; (b) J. B. Hudson, V. Imperial, R. P. Haugland and Z. Diwu, Antiviral Activities of Photoactive Perylenequinones, Photochem. Photobiol., 1997, 65, 352–354; (c) J. Park, D. S. English, Y. Wannemuehler, S. Carpenter and J. W. Petrich, The role of oxygen in the antiviral activity of hypericin and hypocrellin, Photochem. Photobiol., 1998, 68, 593–597.
P. Miskovsky, Hypericin - A New Antiviral and Antitumor Photosensitizer: Mechanism of Action and Interaction with Biological Macromolecules, Curr. Drug Targets, 2002, 3, 55–84; (b) F. Sureau, P. Miskovsky, L. Chinsky and P. Y. Turpin, Hypericin-Induced Cell Photosensitization Involves an Intracellular pH Decrease, J. Am. Chem. Soc., 1996, 118, 9484–9487; (c) M. J. Fehr, M. A. McCloskey and J. W. Petrich, Light-Induced Acidification by the Antiviral Agent Hypericin, J. Am. Chem. Soc., 1995, 117, 1833–1836.
W. D. Lu and W. M. Atkins, A novel antioxidant role for ligand in behavior of glutathione S-transferases: Attenuation of the photodynamic effects of hypericin, Biochemistry, 2004, 43, 12761–12769.
J. B. Hudson, J. Zhou, J. Chen, L. Harris, L. Yip and G. H. N. Towers, Hypocrellin from Hypocrella babuase is phototoxic to human immunodeficiency virus, Photochem. Photobiol., 1994, 60, 253–255; (b) J. Hirayama, K. Ikebuchi, H. Abe, K. W. Kwon, Y. Ohnishi, M. Horiuch, M. Shinagawa, K. Ikuta, N. Kamo and S. Sekiguchi, Photoinactivation of virus infectivity by hypocrellin A, Photochem. Photobiol., 1997, 66, 697–700.
S. Xu, S. Chen, M. Zhang and T. Shen, A novel method for the preparation of amino-substituted hypocrellin B, Bioorg. Med. Chem. Lett., 2001, 11, 2045–2047; (b) S.-S. Qi, X. Lin, M.-M. Zhang, S.-Z. Yan, S.-Q. Yu and S.-L. Chen, Preparation and evaluation of hypocrellin A loaded poly (lactic-co-glycolic acid) nanoparticles for photodynamic therapy, RSC Adv., 2014, 4, 40085–40094; (c) C. Yang, F. Ma, J. Tang, L. Han, S. Wei, L. Zhou, J. Zhou, J. Shen and F. Wang, Synthesis of vanadyl-hypocrellin A complex and its photodynamic properties research, Bioorg. Med. Chem. Lett., 2012, 22, 5003–5007.
C. X. Sun, Y. J. Ma and J. W. Wang, Improved hypocrellin A production in Shiraia bambusicola by light-dark shift, J. Photochem. Photobiol., B, 2018, 182, 100–107; (b) H. N. Murthy, Y. S. Kim, S. Y. Park and K. Y. Paek, Hypericins: Biotechnological production from cell and organ cultures, Appl. Microbiol. Biotechnol., 2014, 98, 9187–9198.
S. Miethbauer, F. Gaube, U. Möllmann, H.-M. Dahse, M. Schmidtke, M. Gareis, M. Pickhardt and B. Liebermann, Antimicrobial, Antiproliferative, Cytotoxic, and Tau Inhibitory Activity of Rubellins and Caeruleoramularin Produced by the Phytopathogenic Fungus Ramularia collo-cygni, Planta Med., 2009, 75, 1523– 1525.
I. Teasdale, M. Waser, S. Wilfert, H. Falk and O. Brüggemann, Photoreactive, water-soluble conjugates of hypericin with polyphosphazenes, Monatsh. Chem., 2012, 143, 355–360.
W. T. Couldwell, A. A. Surnock, A. J. Tobia, B. E. Cabana, C. B. Stillerman, P. A. Forsyth, A. J. Appley, A. M. Spence, D. R. Hinton and T. C. Chen, A Phase 1/2 Study of Orally Administered Synthetic Hypericin for Treatment of Recurrent Malignant Gliomas, Cancer, 2011, 117, 4905– 4915.
D. M. Blake, P.-C. Maness, Z. Huang, E. J. Wolfrum, J. Huang and W. A. Jacoby, Application of the photocatalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells, Sep. Purif. Methods, 1999, 28, 1– 50; (b) N. M. Jukapli and S. Bagheri, Recent developments on titania nanoparticle as photocatalytic cancer cells treatment, J. Photochem. Photobiol., B, 2016, 163, 421–430; (c) R. P. Allaker and K. Memarzadeh, Nanoparticles and the control of oral infections, Int. J. Antimicrob. Agents, 2014, 43, 95–104.
G. D. Cuny, K. D. Landgrebe and T. P. Smith, Photoactivated Virucidal Properties of Tridentate 2,2’-Dihydroxyazobenzene and 2-Salicylideneaminophenol Platinum Pyridine Complexes, Bioorg. Med. Chem. Lett., 1999, 9, 237–240.
D. Park, H. M. Shahbaz, S.-H. Kim, M. Lee, W. Lee, J.-W. Oh, D.-U. Lee and J. Park, Inactivation efficiency and mechanism of UV-TiO2 photocatalysis against murine norovirus using a solidified agar matrix, Int. J. Food Microbiol., 2016, 238, 256–264.
X. Yang and Y. Wang, Photocatalytic effect on plasmid DNA damage under different UV irradiation time, Build. Environ., 2008, 43, 253–257; (b) R. Nakano, H. Ishiguro, Y. Yao, J. Kajioka, A. Fujishima, K. Sunada, M. Minoshima, K. Hashimotod and Y. Kubota, Photocatalytic inactivation of influenza virus by titanium dioxide thin film, Photochem. Photobiol. Sci., 2012, 11, 1293–1298.
F. Vatansever, C. Ferraresi, M. V. P. de Sousa, R. Yin, A. Rineh, S. K. Sharma and M. R. Hamblin, Can biowarfare agents be defeated with light?, Virulence, 2013, 4, 796–825.
C. Ogino, N. Shibata, R. Sasai, K. Takaki, Y. Miyachi, S. Kuroda, K. Ninomiya and N. Shimizu, Construction of protein-modified TiO2 nanoparticles for use with ultrasound irradiation in a novel cell injuring method, Bioorg. Med. Chem. Lett., 2010, 20, 5320–5325.
M. R. Hamblin, Potentiation of antimicrobial photodynamic inactivation by inorganic salts, Expert Rev. Anti Infect. Ther., 2017, 15, 1059–1069; (b) M. R. Hamblin and H. Abrahamse, Inorganic Salts and Antimicrobial Photodynamic Therapy: Mechanistic Conundrums?, Molecules, 2018, 23, 3190, DOI: 10.3390/molecules23123190.
M. E. Lim, Y.-L. Lee, Y. Zhang and J. J. H. Chu, Photodynamic inactivation of viruses using upconversion nanoparticles, Biomaterials, 2012, 33, 1912–1920.
S. H. Friedman, D. L. DeCamp, R. P. Sijbesma, G. Srdanov, F. Wudl and G. L. Kenyon, Inhibition of the HIV-1 Protease by Fullerene Derivatives: Model Building Studies and Experimental Verification, J. Am. Chem. Soc., 1993, 115, 6506–6509.
F. Käsermann and C. Kempf, Buckminsterfullerene and Photodynamic Inactivation of Viruses, Rev. Med. Virol., 1998, 8, 143–151; (b) P. Mroz, G. P. Tegos, H. Gali, T. Wharton, T. Sarnad and M. R. Hamblin, Photodynamic therapy with fullerenes, Photochem. Photobiol. Sci., 2007, 6, 1139–1149; (c) S. K. Sharma, L. Y. Chiang and M. R. Hamblin, Photodynamic therapy with fullerenes in vivo: reality or a dream?, Nanomedicine, 2011, 6, 1813– 1825.
Z. Markovic and V. Trajkovic, Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60), Biomaterials, 2008, 29, 3561–3573.
Y. Yamakoshi, N. Umezawa, A. Ryu, K. Arakane, N. Miyata, Y. Goda, M. Toshiki and T. Nagano, Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2•− versus 1O2, J. Am. Chem. Soc., 2003, 125, 12803–12809.
V. V. Zarubaev, I. Belousova, V. Rylkov, A. Slita, A. Sirotkin, P. Anfimov, T. Muraviova and A. Starodubtsev, Photodynamic Inactivation of Enveloped Viruses by Fullerene: Study of Efficacy and Safety, in Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes, ed. F. Cataldo and T. Da Ros, Springer, Heidelberg, 2008, pp. 107–120; (b) Y. Rud, S. Prylutska, L. Buchatskyy, Y. Prylutskyy, U. Ritter and P. Scharff, Photodynamic inactivation of mosquito iridovirus (MIV) by C60 fullerene, Materialwiss. Werkstofftech., 2011, 42, 136–138; (c) Y. Rud, L. Buchatskyy, Y. Prylutskyy, O. Marchenko, A. Senenko, C. Schütze and U. Ritter, Using C60 fullerenes for photodynamic inactivation of mosquito iridescent viruses, J. Enzyme Inhibit. Med. Chem., 2012, 27, 614–617.
L. J. dos Santos, R. B. Alves, R. P. de Freitas, J.-F. Nierengarten, L. E. F. Magalhães, K. Krambrock and M. V. B. Pinheiro, Production of reactive oxygen species induced by a new [60]fullerene derivative bearing a tetrazole unit and its possible biological applications, J. Photochem. Photobiol., A, 2008, 200, 277–281.
J. Lee, Y. Mackeyev, M. Cho, D. Li, J.-H. Kim, L. J. Wilson and P. J. J. Alvarez, Photochemical and Antimicrobial Properties of Novel C60 Derivatives in Aqueous Systems, Environ. Sci. Technol., 2009, 43, 6604–6610.
A. R. Badireddy, J. F. Budarz, S. Chellam and M. R. Wiesner, Bacteriophage Inactivation by UV-A Illuminated Fullerenes: Role of Nanoparticle-Virus Association and Biological Targets, Environ. Sci. Technol., 2012, 46, 5963–5970.
M. Y. Eropkin, E. Y. Melenevskaya, K. V. Nasonova, T. S. Bryazzhikova, E. M. Eropkina, D. M. Danilenko and O. I. Kiselev, Synthesis and biological activity of fullerenols with various contents of hydroxyl groups, Pharm. Chem. J., 2013, 47, 87–91.
M. Cho, J. Lee, Y. Mackeyev, L. J. Wilson, P. J. J. Alvarez, J. B. Hughes and J.-H. Kim, Visible Light Sensitized Inactivation of MS-2 Bacteriophage by a Cationic Amine-Functionalized C60 Derivative, Environ. Sci. Technol., 2010, 44, 6685–6691; (b) J. Lee, Y. Mackeyev, M. Cho, L. J. Wilson, J.-H. Kim and P. J. J. Alvarez, C60 Aminofullerene Immobilized on Silica as a Visible-Light-Activated Photocatalyst, Environ. Sci. Technol., 2010, 44, 9488–9495.
S. D. Snow, K. E. Park and J.-H. Kim, Cationic Fullerene Aggregates with Unprecedented Virus Photoinactivation Efficiencies in Water, Environ. Sci. Technol. Lett., 2014, 1, 290–294.
S. Tanimoto, S. Sakai, S. Matsumura, D. Takahashi and K. Toshima, Target-selective photo-degradation of HIV-1 protease by a fullerene-sugar hybrid, Chem. Commun., 2008, 5767–5769; (b) S. Tanimoto, S. Sakai, E. Kudo, S. Okada, S. Matsumura, D. Takahashi and K. Toshima, Target-Selective Photodegradation of HIV-1 Protease and Inhibition of HIV-1 Replication in Living Cells by Designed Fullerene–Sugar Hybrids, Chem. – Asian J., 2012, 7, 911–914.
I. M. Belousova, O. B. Danilov, T. D. Muraveva, I. M. Kiselyakov, V. V. Rylkov, T. K. Krisko, O. I. Kiselev, V. V. Zarubaev, A. K. Sirotkin and L. B. Piotrovskii, Solid-phase photosensitizers based on fullerene C60 for photo-dynamic inactivation of viruses in biological liquids, J. Opt. Technol., 2009, 76, 243–250; (b) I. M. Belousova, I. M. Kislyakov, T. D. Muraviova, A. M. Starodubtsev, T. K. Krisko, E. A. Selivanov, N. P. Sivakova, I. S. Golovanova, S. D. Volkova, A. A. Shtro and V. V. Zarubaev, Photodynamic inactivation of enveloped virus in protein plasma preparations by solid-phase fullerene-based photosensitizer, Photodiagn. Photodyn. Ther., 2014, 11, 165–170.
K. J. Moor, D. C. Valle, C. Li and J.-H. Kim, Improving the Visible Light Photoactivity of Supported Fullerene Photocatalysts through the Use of [C70] Fullerene, Environ. Sci. Technol., 2015, 49, 6190–6197.
K. J. Moor, C. O. Osuji and J.-H. Kim, Dual-Functionality Fullerene and Silver Nanoparticle Antimicrobial Composites via Block Copolymer Templates, ACS Appl. Mater. Interfaces, 2016, 8, 33583– 33591; (b) C. Constantin, M. Neagu, R.-M. Ion, M. Gherghiceanu and C. Stavaru, Fullerene–porphyrin nanostructures in photodynamic therapy, Nanomedicine, 2010, 5, 307–317; (c) M. Helmreich, E. A. Ermilov, M. Meyer, N. Jux, A. Hirsch and B. Röder, Dissipation of Electronic Excitation Energy within a C60 [6: 0]-Hexaadduct Carrying 12 Pyropheophorbide a Moieties, J. Am. Chem. Soc., 2005, 127, 8376–8385; (d) F. Rancan, M. Helmreich, A. Mölich, N. Jux, A. Hirsch, B. Röder, C. Witt and F. Böhm, Fullerene-pyropheophorbide a complexes as sensitizer for photodynamic therapy: Uptake and photo-induced cytotoxicity on Jurkat cells, J. Photochem. Photobiol., B, 2005, 80, 1–7.
X. Hu, L. Mu, J. Wen and Q. Zhou, Covalently synthesized graphene oxide-aptamer nanosheets for efficient visible-light photocatalysis of nucleic acids and proteins of viruses, Carbon, 2012, 50, 2772–2781.
O. Akhavan, M. Choobtashani and E. Ghaderi, Protein Degradation and RNA Efflux of Viruses Photocatalyzed by Graphene–Tungsten Oxide Composite Under Visible Light Irradiation, J. Phys. Chem. C, 2012, 116, 9653–9659.
A. R. Deokar, A. P. Nagvenkar, I. Kalt, L. Shani, Y. Yeshurun, A. Gedanken and R. Sarid, Graphene-Based “Hot Plate” for the Capture and Destruction of the Herpes Simplex Virus Type 1, Bioconjugate Chem., 2017, 28, 1115– 1122.
B. Ziem, J. Rahn, I. Donskyi, K. Silberreis, L. Cuellar, J. Dernedde, G. Keil, T. C. Mettenleiter and R. Haag, Polyvalent 2D Entry Inhibitors for Pseudorabies and African Swine Fever Virus, Macromol. Biosci., 2017, 1600499.
E. S. Nyman and P. H. Hynninen, Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy, J. Photochem. Photobiol., B, 2004, 73, 1–28.
M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, The role of porphyrin chemistry in tumor imaging and photodynamic therapy, Chem. Soc. Rev., 2011, 40, 340–362.
J. Kou, D. Dou and L. Yang, Porphyrin photosensitizers in photodynamic therapy and its applications, Oncotarget, 2017, 8, 81591–81603.
K. T. de Oliveira, P. B. Momo, F. F. de Assis, M. A. B. Ferreira and T. J. Brocksom, Chlorins: Natural Sources, Synthetic Developments and Main Applications, Curr. Org. Synth., 2014, 11, 42–58.
A. M. Durantini, D. A. Heredia, J. E. Durantini and E. N. Durantini, BODIPYs to the rescue: Potential applications in photodynamic inactivation, Eur. J. Med. Chem., 2018, 144, 651–661; (b) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, BODIPY dyes in photodynamic therapy, Chem. Soc. Rev., 2013, 42, 77–88; (c) S. G. Awuah and Y. You, Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy, RSC Adv., 2012, 2, 11169–11183.
A. Turksoy, D. Yildiz and E. U. Akkaya, Photosensitization and controlled photosensitization with BODIPY dyes, Coord. Chem. Rev., 2019, 379, 47–64.
M. T. Wan and J. Y. Lin, Current evidence and applications of photodynamic therapy in dermatology, Clin. Cosmet. Investig. Dermatol., 2014, 7, 145–163.
M. O. Senge and J. C. Brandt, Temoporfin (Foscan®, 5,10,15,20-Tetra(m-hydroxyphenyl)chlorin) – A Second-generation Photosensitizer, Photochem. Photobiol., 2011, 87, 1240–1296; (b) M. O. Senge, mTHPC – A drug on its way from second to third generation photosensitizer?, Photodiagn. Photodyn. Ther., 2012, 9, 170–179.
C. Fenton and C. M. Perry, Verteporfin: a review of its use in the management of subfoveal choroidal neovascularisation, Drugs Aging, 2006, 23, 421–445.
I. Stojiljkovic, B. D. Evavold and V. Kumar, Antimicrobial properties of porphyrins, Expert Opin. Invest. Drugs, 2003, 10, 309–320.
E. Alves, M. A. F. Faustino, M. G. P. M. S. Neves, Â. Cunha, H. Nadais and A. Almeida, Potential applications of porphyrins in photodynamic inactivation beyond the medical scope, J. Photochem. Photobiol., C, 2015, 22, 34–57.
D. V. Titov, M. L. Gening, Y. E. Tsvetkov and N. E. Nifantiev, Glycoconjugates of porphyrins with carbohydrates: methods of synthesis and biological activity, Russ. Chem. Rev., 2014, 83, 523–554; (b) C. Moylan, E. M. Scanlan and M. O. Senge, Chemical Synthesis and Medicinal Applications of Glycoporphyrins, Curr. Med. Chem., 2015, 22, 2238–2348.
N. Malatesti, I. Munitic and I. Jurak, Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial and immunosuppressive agents, Biophys. Rev., 2017, 9, 149–168.
P. Calzavara-Pinton, M. Arisi, E. Sereni and B. Ortel, A Critical Reappraisal of Off-Label Indications for Topical Photodynamic Therapy with Aminolevulinic Acid and Methylaminolevulinate, Rev. Recent Clin. Trials, 2010, 5, 112–116.
J. P. C. Tomé, E. M. P. Silva, A. M. V. M. Pereira, C. M. A. Alonso, M. A. F. Faustino, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, S. A. P. Tavares, R. R. Duarte, M. F. Caeiro and M. L. Valdeira, Synthesis of neutral and cationic tripyridylporphyrin-D-galactose conjugates and the photoinactivation of HSV-1, Bioorg. Med. Chem., 2007, 15, 4705–4713.
K. Zupán, M. Egyeki, K. Tóth, A. Fekete, L. Herényi, K. Módos and G. Csík, Comparison of the efficiency and the specificity of DNA-bound and free cationic porphyrin in photodynamic virus inactivation, J. Photochem. Photobiol., B, 2008, 90, 105–112.
L. Costa, E. Alves, C. M. B. Carvalho, J. P. C. Tomé, M. A. F. Faustino, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, A. Cunha and A. Almeida, Sewage bacteriophage photoinactivation by cationic porphyrins: a study of charge effect, Photochem. Photobiol. Sci., 2008, 7, 415–422; (b) C. M. B. Carvalho, J. P. C. Tomé, M. A. F. Faustino, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, L. Costa, E. Alves, A. Oliveira, Â. Cunha and A. Almeida, Antimicrobial photodynamic activity of porphyrin derivatives: Potential application on medical and water disinfection, J. Porphyrins Phthalocyanines, 2009, 13, 574–577; (c) J. S. Cardoso-Rurr, J. P. de Paiva, I. G. Paulino-Lima, T. A. M. de Alencar, C. A. S. Lage and A. C. Leitão, Microbiological Decontamination of Water: Improving the Solar Disinfection Technique (SODIS) with the Use of Nontoxic Vital Dye Methlene Blue, Photochem. Photobiol., 2018, 95, 618–626.
L. Costa, J. P. C. Tomé, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, M. A. F. Faustino, Â. Cunha, N. C. M. Gomes and A. Almeida, Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of aPDT, Antiviral Res., 2011, 91, 278–282.
L. Costa, J. P. C. Tomé, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, Â. Cunha, M. A. F. Faustino and A. Almeida, Susceptibility of non-enveloped DNA- and RNA-type viruses to photodynamic inactivation, Photochem. Photobiol. Sci., 2012, 11, 1520–1523.
L. Costa, A. C. Esteves, A. Correia, C. Moreirinha, I. Delgadillo, Â. Cunha, M. G. P. S. Neves, M. A. F. Faustino and A. Almeida, SDS-PAGE and IR spectroscopy to evaluate modifications in the viral protein profile induced by a cationic porphyrinic photosensitizer, J. Virol. Methods, 2014, 209, 103–109.
B. L. Carpenter, F. Scholle, H. Sadeghifar, A. J. Francis, J. Boltersdorf, W. W. Weare, D. S. Argyropoulos, P. A. Maggard and R. A. Ghiladi, Synthesis, Characterization, and Antimicrobial Efficacy of Photomicrobicidal Cellulose Paper, Biomacromolecules, 2015, 16, 2482–2492.
B. A. Cohen, A. E. Kaloyeros and M. Bergkvist, Nucleotide-driven packaging of a singlet oxygen generating porphyrin in an icosahedral virus, J. Porphyrins Phthalocyanines, 2012, 16, 47–54.
S. L. Stanley, F. Scholle, J. Zhu, Y. Lu, X. Zhang, X. Situ and R. A. Ghiladi, Photosensitizer-Embedded Polyacrylonitrile Nanofibers as Antimicrobial Non-Woven Textile, Nanomaterials, 2016, 6, 77, DOI: 10.3390/nano6040077.
M. Merchat, G. Bertolini, P. Giacomini, A. Villanueva and G. Jori, Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria, J. Photochem. Photobiol., B, 1996, 32, 153– 157; (b) L. L. Trannoy, Y. Van Hensbergen, J. W. M. Lagerberg and A. Brand, Photodynamic treatment with mono-phenyl-tri-(N-methyl-4-pyridyl)-porphyrin for pathogen inactivation in cord blood stem cell products, Transfusion, 2008, 48, 2629–2637.
M. J. Casteel, K. Jayaraj, A. Gold, L. M. Ball and M. D. Sobsey, Photoinactivation of Hepatitis A Virus by Synthetic Porphyrins, Photochem. Photobiol., 2004, 80, 294–300.
C. Kasturi and M. S. Platz, Inactivation of Lambda Phage with 658 nm Light Using a DNA binding Porphyrin Photosensitizer, Photochem. Photobiol., 1992, 56, 427–429; (b) H. Majiya, O. O. Adeyemi, M. Herod, N. J. Stonehouse and P. Millner, Photodynamic inactivation of non-enveloped RNA viruses, J. Photochem. Photobiol., B, 2018, 189, 87–94.
L. L. Trannoy, J. W. M. Lagerberg, T. M. A. R. Dubbelman, H. J. Schuitmaker and A. Brand, Positively charged porphyrins: a new series of photosensitizers for sterilization of RBCs, Transfusion, 2004, 44, 1186–1196; (b) L. L. Trannoy, F. G. Terpstra, D. de Korte, J. W. M. Lagerberg, A. J. Verhoeven, A. Brand and F. A. C. van Engelenburg, Differential sensitivities of pathogens in red cell concentrates to Tri-P(4)-photoinactivation, Vox Sang., 2006, 91, 111–118.
J. P. C. Tomé, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, A. F. Mendonça, I. N. Pegado, R. Duarte and M. L. Valdeira, Synthesis of glycoporphyrin derivatives and their antiviral activity against herpes simplex virus types 1 and 2, Bioorg. Med. Chem., 2003, 13, 3878– 3888.
L. A. Pedigo, A. J. Gibbs, R. J. Scott and C. N. Street, Absence of bacterial resistance following repeat exposure to photodynamic therapy, Proc. SPIE, 2009, 7380, 73803H, DOI: 10.1117/12.822834; (b) A. Tavares, C. M. B. Carvalho, M. A. Faustino, M. G. P. M. S. Neves, J. P. C. Tomé, A. C. Tomé, J. A. S. Cavaleiro, A. Cunha, N. C. M. Gomes, E. Alves and A. Almeida, Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment, Mar. Drugs, 2010, 8, 91–105; (c) G. P. Tegos and M. R. Hamblin, Phenothiazinium Antimicrobial Photosensitizers Are Substrates of Bacterial Multidrug Resistance Pumps, Antimicrob. Agents Chemother., 2006, 50, 196–203.
F. Vargas, C. Rivas, T. Zoltan, L. Padrón, C. Izzo, V. López, L. Gómez, F. Pujol, H. Rangel, D. Garzaro and R. Fabbro, Comparative antiviral (HIV) photoactivity of metalized meso-tetraphenylsulfonated porphyrins, Med. Chem., 2008, 4, 138–145.
J. Dairou, C. Vever-Bizet and D. Brault, Interaction of sulfonated anionic porphyrins with HIV glycoprotein gp120: photodamages revealed by inhibition of antibody binding to V3 and C5 domains, Antiviral Res., 2004, 61, 37–47.
A. V. Teles, T. M. A. Oliveira, F. C. Bezerra, L. Alonso, A. Alonso, I. E. Borissevitch, P. J. Gonçalves and G. R. L. Souza, Photodynamic inactivation of bovine herpesvirus type 1 (Bohv-1) by porphyrins, J. Gen. Virol., 2018, 99, 1301–1306.
R. M. Ion and M. A. Calin, Comparative study of some nano- and micro-sensitizers in photodynamic inactivation of microorganisms, J. Optoelectron. Adv. Mater., 2007, 9, 1933–1938.
K.-K. Wang, B.-J. Kim, S.-H. Ko, D. H. Choi and Y.-R. Kim, Fabrication of Photofunctional Nanoporous Membrane and Its Photoinactivation Effect of Vesicular Stomatitis Virus, J. Nanomater., 2012, 454507, DOI: 10.1155/2012/454507.
B. S. T. Peddinti, F. Scholle, R. A. Ghiladi and R. J. Spontak, Photodynamic Polymers as Comprehensive Anti-Infective Materials: Staying Ahead of a Growing Global Threat, ACS Appl. Mater. Interfaces, 2018, 10, 25955–25959.
L. Negosanti, V. Pinto, R. Sgarzani, F. Negosanti, G. Zannetti and R. Cipriani, Photodynamic therapy with topical aminolevulinic acid, World J. Dermatol., 2014, 3, 6– 14.
M. Kim, H. Y. Jung and H. J. Park, Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications, Int. J. Mol. Sci., 2015, 16, 23259–23278.
P. Babilas, S. Karrer, A. Sidoroff, M. Landthaler and R.-M. Szeimies, Photodynamic therapy in dermatology – an update, Photodermatol., Photoimmunol. Photomed., 2005, 21, 142–149; (b) F. Harris and L. Pierpoint, Photodynamic Therapy Based on 5-Aminolevulinic Acid and Its Use as an Antimicrobial Agent, Med. Res. Rev., 2012, 32, 1292–1327; (c) P. Babilas, S. Schreml, M. Landthaler and R.-M. Szeimies, Photodynamic therapy in dermatology: state-of-the-art, Photodermatol., Photoimmunol. Photomed., 2010, 26, 118–132.
R. Ammann, T. Hunziker and L. R. Braathen, Topical photodynamic therapy in verrucae. A pilot study, Dermatology, 1995, 191, 346–347.
Z. Smetana, Z. Malik, A. Orenstein, E. Mendelson and E. Ben-Hur, Treatment of Viral Infections With 5-Aminolevulinic Acid and Light, Lasers Surg. Med., 1997, 21, 351–358.
E. V. Ross, R. Romero, N. Kollias, C. Crum and R. R. Anderson, Selectivity of protoporphyrin IX fluorescence for condylomata after topical application of 5-aminolaevulinic acid: implications for photodynamic treatment, Br. J. Dermatol., 1997, 137, 736–742.
K. Lang, P. Lehmann, K. Bolsen, T. Ruzicka and C. Fritsch, Aminolevulinic acid: pharmacological profile and clinical indication, Expert Opin. Invest. Drugs, 2001, 10, 1139–1156.
S. H. Ibbotson, Topical 5-aminolaevulinic acid photodynamic therapy for the treatment of skin conditions other than non-melanoma skin cancer, Br. J. Dermatol., 2002, 146, 178–188.
R.-M. Szeimies, M. Landthaler and S. Karrer, Non-oncologic indications for ALA-PDT, J. Dermatol. Treat., 2002, 13(Suppl. 1), s13–s18.
S. C. Kanick, S. C. Davis, Y. Zhao, K. L. Sheehan, T. Hasan, E. V. Maytin, B. W. Pogue and M. S. Chapman, Pre-treatment protoporphyrin IX concentration in actinic keratosis lesions may be a predictive biomarker of response to aminolevulinic-acid based photodynamic therapy, Photodiagn. Photodyn. Ther., 2015, 12, 561–566; (b) Information about Ameluz® on the EMA homepage: European Medicines Agency (2018), Ameluz (5-aminolaevulinic acid) (Pub. No. EMA/121475/2018), retrieved March 29, 2019, from https://www.ema.europa.eu/en/documents/overview/ameluz-epar-medicine-overview_en.pdf.
M. H. Gold and A. Moiin, Treatment of Verrucae Vulgaris and Molluscum Contagiosum with Photodynamic Therapy, Dermatol. Clin., 2007, 25, 75–80.
I.-M. Stender, F. M. Borgbjerg, J. Villumsen, J. Lock-Andersen and H.-C. Wulf, Pain induced by photodynamic therapy of warts, Photodermatol., Photoimmunol. Photomed., 2006, 22, 304–309.
E. Mulhem and S. Pinelis, Treatment of Nongenital Cutaneous Warts, Am. Fam. Physician, 2011, 84, 288–293.
Y. G. Lu, J. J. Wu, Y. He, H. Z. Yang and Y. D. Yang, Efficacy of topical aminolaevulinic acid photodynamic therapy for the treatment of verruca planae, Photomed. Laser Surg., 2010, 28, 561–563; (b) R. Fathi and M. M. Tsoukas, Genital warts and other HPV infections: Established and novel therapies, Clinics Dermatol., 2014, 32, 299–306.
W.-S. Chong and G. Y. M. Kang, Dramatic clearance of a recalcitrant acral viral wart using methyl aminolevulinate-red light photodynamic therapy, Photodermatol., Photoimmunol. Photomed., 2009, 25, 225–226; (b) P. Soergel, X. Wang, H. Stepp, H. Hertel and P. Hillemanns, Photodynamic therapy of cervical intraepithelial neoplasia with hexaminolevulinate, Lasers Surg. Med., 2008, 40, 611–615.
J. E. Kim, S. J. Kim, J. I. Hwang, K. Lee, H. Park and B. K. Cho, New proposal for the treatment of viral warts with intralesional injection of 5-aminolevulinic acid photodynamic therapy, J. Dermatol. Treat., 2012, 23, 192– 195.
M. Fernández-Guarino, A. Harto and P. Jaén, Treatment of recalcitrant viral warts with pulsed dye laser MAL-PDT, J. Dermatol. Treat., 2011, 22, 226–228; (b) M. F. Guarino, A. Harto and P. Jaén, Treatment of Recalcitrant Viral Warts with Photodynamic Therapy with Mal and Red Light, J. Cosmet., Dermatol. Sci. Appl., 2013, 3, 117–120.
A. Ohtsuki, T. Hasegawa, Y. Hirasawa, H. Tsuchhasi and S. Ikeda, Photodynamic therapy using light-emitting diodes for the treatment of viral warts, J. Dermatol., 2009, 36, 525–528.
Y. S. Wang, Y. K. Tay, C. Kwok and E. Tan, Photodynamic therapy with 20% aminolevulinic acid for the treatment of recalcitrant viral warts in an Asian population, Int. J. Dermatol., 2007, 46, 1180–1184.
I.-M. Stender, R. Na, H. Fogh, C. Gluud and H. C. Wulf, Photodynamic therapy with 5-aminolevulinic acid or placebo for recalcitrant foot and hand warts: randomised double-blind trial, Lancet, 2000, 355, 963–966.
S. M. Fuchs, J. W. Fluhr, L. Bankova, J. Tittelbach, G. Hoffmann and P. Elsner, Photodynamic therapy (PDT) and waterfiltered infrared A (wIRA) in patients with recalcitrant common hand and foot warts, Germ. Med. Sci., 2004, 2, doc08.
C. A. Schroeter, L. Kaas, J. J. Waterval, P. M. Bos and H. A. Neumann, Successful treatment of periungual warts using photodynamic therapy: a pilot study, J. Eur. Acad. Dermatol. Venereol., 2007, 21, 1170–1174.
G. Qian, S. Wang, D. Deng and G. Yang, Is the step-up therapy of topical 5-aminolevulinic acid photodynamic therapy effective and safe for the patients with recalcitrant facial flat wart?, Dermatol. Ther., 2014, 27, 83–88.
M. Caucanas, P. Gillard and O. Vanhooteghem, Efficiency of Photodynamic Therapy in the Treatment of Diffuse Facial Viral Warts in an Immunosuppressed Patient: Towards a Gold Standard?, Case Rep. Dermatol., 2010, 2, 207–213.
M.-Y. Lin and L.-H. Xiang, Topical 5-aminolevulinic acid photodynamic therapy for recalcitrant facial flat wart in Chinese subjects, J. Dermatol., 2008, 35, 658–661.
H.-W. Wang, X.-L. Wang, L.-L. Zhang, M.-X. Guo and Z. Huang, Aminolevulinic acid (ALA)-assisted photodynamic diagnosis of subclinical and latent HPV infection of external genital region, Photodiagn. Photodyn. Ther., 2008, 5, 251–255; (b) G. Gross, H. Ikenberg, K. U. Petry, H. Pfister, P. Schneede, H. Schöfer and R.-M. Szeimies, Guideline for Condylomata acuminata and other HPV-associated diseases of the genitals, the anus and the urethra, J. Dtsch. Dermatol. Ges., 2008, 6, 153–162; (c) H.-W. Wang, L.-L. Zhang, F. Miao, T. Lv, X.-L. Wang and Z. Huang, Treatment of HPV Infection-Associated Cervical Condylomata Acuminata with 5-Aminolevulinic Acid-Mediated Photodynamic Therapy, Photochem. Photobiol., 2012, 88, 565–569; (d) N. M. Inada, M. M. da Costa, O. C. C. Guimarães, E. da Silva Ribeiro, C. Kurachi, S. M. Quintana, W. Lombardi and V. S. Bagnato, Photodiagnosis and treatment of condyloma acuminatum using 5-aminolevulinic acid and homemade devices, Photodiagn. Photodyn. Ther., 2012, 9, 60–68; (e) C. Ao, J. Xie, L. Wang, S. Li, L. Jiang, H. Liu and K. Zeng, 5-Aminolevulinic acid and photodynamic therapy for anal canal condyloma acuminatum: A series of 19 cases and literature review, Photodiagn. Photodyn. Ther., 2018, 23, 230–234.
J. Xu, L. Xiang, J. Chen, Q. He, Q. Li, J. Li and J. Wang, The combination treatment using CO2 laser and photodynamic therapy for HIV seropositive men with intraanal warts, Photodiagn. Photodyn. Ther., 2013, 10, 186–193.
P. Goon, C. Sonnex, P. Jani, M. Stanley and H. Sudhoff, Recurrent respiratory papillomatosis: An overview of current thinking and treatment, Eur. Arch. Otorhinolaryngol., 2008, 2, 147–151; (b) N. N. Venkatesan, H. S. Pine and M. P. Underbrink, Recurrent Respiratory Papillomatosis, Otolaryngol. Clin. North Am., 2012, 45, 671–694; (c) Z. Hu, L. Liu, W. Zhang, H. Liu, J. Li, L. Jiang and K. Zeng, Dynamics of HPV viral loads reflect the treatment effect of photodynamic therapy in genital warts, Photodiagn. Photodyn. Ther., 2018, 21, 86–90; (d) S. Hu, Y. Yang, B. Jiang, D. Su, L. Zhang, Z. Huang and F. Zhang, Treatment of condyloma acuminatum using the combination of laser ablation and ALA-PDT, Photodiagn. Photodyn. Ther., 2019, 25, 193–196.
D. Kacerovska, K. Pizinger, M. Kumpova and P. Cetkovska, Genital Warts Treated By Photodynamic Therapy, Skinmed, 2007, 6, 295–297.
H. Shi, X. Zhang, C. Ma, N. Yu, J. Wang, L. Xia, X. Ge, M. Liu and A. Duan, Clinical Analysis of Five Methods Used to Treat Condylomata Acuminata, Dermatology, 2013, 227, 338–345.
X.-L. Wang, H.-W. Wang, Z. Huang, H. Stepp, R. Baumgartner, C. Dannecker and P. Hillemanns, Study of Protoporphyrin IX (PpIX) Pharmacokinetics After Topical Application of 5-Aminolevulinic Acid in Urethral Condylomata Acuminata, Photochem. Photobiol., 2007, 83, 1069–1073.
N. M. Inada, C. Kurachi, J. Ferreira, E. S. Ribeiro, O. C. C. Guimarães, S. M. Quintana, W. Lombardi and V. S. Bagnato, Treatment of vulvar/vaginal condyloma by HPV: developed instrumentation and clinical report, Proc. SPIE, 2009, 7380, 738054.
C. A. Morton, R.-M. Szeimies, A. Sidoroff and L. R. Braathen, European guidelines for topical photodynamic therapy part 2: emerging indications – field cancerization, photorejuvenation and inflammatory/infective dermatoses, J. Eur. Acad. Dermatol. Venereol., 2013, 27, 672–679.
K. Chen, B. Z. Chang, M. Ju, X. H. Zhang and H. Gu, Comparative study of photodynamic therapy vs. CO2 laser vaporization in treatment of condylomata acuminata, a randomized clinical trial, Br. J. Dermatol., 2007, 156, 516–520.
J. Liang, X. N. Lu, H. Tang, Z. Zhang, J. Fan and J. H. Xu, Evaluation of photodynamic therapy using topical aminolevulinic acid hydrochloride in the treatment of condylomata acuminata: a comparative, randomized clinical trial, Photodermatol., Photoimmunol. Photomed., 2009, 25, 293– 297.
R.-M. Szeimies, V. Schleyer, I. Moll, M. Stocker, M. Landthaler and S. Karrer, Adjuvant photodynamic therapy does not prevent recurrence of condylomata acuminata after carbon dioxide laser ablation - A phase III, prospective, randomized, bicentric, double-blind study, Dermatol. Surg., 2009, 35, 757–764.
V. Nucci, D. Torchia and P. Cappugi, Treatment of anogenital condylomata acuminata with topical photodynamic therapy: report of 14 cases and review, Int. J. Infect. Dis., 2010, 14S, e280–e282.
X. Mi, W. Chai, H. Zheng, Y.-G. Zuo and J. Li, A randomized clinical comparative study of cryotherapy plus photodynamic therapy vs. cryotherapy in the treatment of multiple condylomata acuminata, Photodermatol., Photoimmunol. Photomed., 2011, 27, 176–180.
Z. Zhang, X.-N. Lu, J. Liang, H. Tang, Y.-S. Yang, X.-H. Zhu, J. Du, Y.-Y. Shen and J.-H. Xu, Evaluation of photodynamic therapy using topical aminolevulinic acid hydrochloride in the treatment of condylomata acuminate, Int. J. Clin. Exp. Med., 2015, 8, 6517–6521.
G.-Y. Chu, T. C.-C. Chang and C.-H. Chang, Buschke-Löwenstein tumor (giant condyloma acuminatum) successfully treated by topical photodynamic therapy: a case report, Dermatol. Sin., 2013, 31, 94–97.
Z.-Y. Bu, X.-H. Yu, L.-M. Wu, J.-B. Zhong, P. Yang and J. Chen, Normalization of regulatory T cells, serum TGF-β1, and LTN after 5-aminolevulinic acid-photodynamic therapy in patients with condyloma acuminate, Exp. Ther. Med., 2017, 13, 3327–3332.
X. Li, X. Wang, J. Gu, Y. Ma, Z. Liu and Y. Shi, Needle-free injection of 5-aminolevulinic acid in photodynamic therapy for the treatment of condylomata acuminata, Exp. Ther. Med., 2013, 6, 236–240.
X.-L. Wang, H.-W. Wang, M.-X. Guo and Z. Huang, Combination of immunotherapy and photodynamic therapy in the treatment of Bowenoid papulosis, Photodiagn. Photodyn. Ther., 2007, 4, 88–93; (b) M. Ziada, C. B. Bunker and A. Muneer, Premalignant penile lesions, J. Clin. Urol., 2016, 9, 216–222.
X. Yu and H. Zheng, Infections after photodynamic therapy in Condyloma acuminatum patients: incidence and management, Environ. Sci. Pollut. Res., 2018, 25, 14000–14005.
S. Varada, M. Posnick, D. Alessa and M. K. Ramírez-Fort, Management of Cutaneous Human Papillomavirus Infection in Immunocompromised Patients, Curr. Probl. Dermatol., 2014, 45, 197–215; (b) F. Granel-Brocard, J. F. Cuny and J. L. Schmutz, Efficacy of photodynamic therapy (PDT) in a recalcitrant wart in an immunodeficient subject, Eur. J. Dermatol., 2008, 18, 601; (c) D. Krüger-Corcoran, E. Stockfleth, J. S. Jürgensen, A. Maltusch, I. Nindl, W. Sterry, B. Lange-Asschenfeldt and C. Ulrich, Humane Papillomvirus-assoziierte Warzen bei organtransplantierten Patienten: Inzidenz, Risikofaktoren, Management, Hautarzt, 2010, 61, 220– 229.
I. Nindl, M. Gottschling and E. Stockfleth, Human papillomaviruses and non-melanoma skin cancer: basic virology and clinical manifestations, Dis. Markers, 2007, 23, 247–259.
S. Daayana, U. Winters, P. L. Stern and H. C. Kitchener, Clinical and immunological response to photodynamic therapy in the treatment of vulval intraepithelial neoplasia, Photochem. Photobiol. Sci., 2011, 10, 802–809.
L. Hampson, P. Martin-Hirsch and I. N. Hampson, An overview of early investigational drugs for the treatment of human papilloma virus infection and associated dysplasia, Expert Opin. Invest. Drugs, 2015, 24, 1529–1537.
Q. Guo, B. Dong, F. Nan, D. Guan and Y. Zhang, 5-Aminolevulinic acid photodynamic therapy in human cervical cancer via the activation of microRNA-143 and suppression of the Bcl-2/Bax signaling pathway, Mol. Med. Rep., 2016, 14, 544–550; (b) Y. Fu, Y. Bao, Y. Hui, X. Gao, M. Yang and J. Chang, Topical photodynamic therapy with 5-aminolevulinic acid for cervical high-risk HPV infection, Photodiagn. Photodyn. Ther., 2016, 13, 29–33; (c) Z. Hu, J. Li, H. Liu, L. Jiang and K. Zeng, Treatment of latent or subclinical Genital HPV Infection with 5-aminolevulinic acid-based photodynamic therapy, Photodiagn. Photodyn. Ther., 2018, 23, 362–364.
P. Soergel, G. F. Dahl, M. Onsrud and P. Hillemanns, Photodynamic Therapy of Cervical Intraepithelial Neoplasia 1–3 and Human Papillomavirus (HPV) Infection With Methylaminolevulinate and Hexaminolevulinate – A Double-Blind, Dose-Finding Study, Lasers Surg. Med., 2012, 44, 468–474.
E.-S. Abdel-Hady, P. Martin-Hirsch, M. Duggan-Keen, P. L. Stern, J. V. Moore, G. Corbitt, H. C. Kitchener and I. N. Hampson, Immunological and Viral Factors Associated with the Response of Vulval Intraepithelial Neoplasia to Photodynamic Therapy, Cancer Res., 2001, 61, 192–196.
I. Ballester, I. Belinchón, J. Guijarro, F. Oltra, F. Toledo and L. Cuesta, Photodynamic therapy of vulvar intraepithelial neoplasia using methyl aminolevulinate, J. Dermatol. Treat., 2012, 23, 156–158; (b) P. Schleier, P. Hyckel, A. Berndt, H.-P. Bode, V. Albrecht, W. Hindermann, H. Kosmehl, W. Zenk and D. Schumann, Photodynamic therapy of virus-associated epithelial tumours of the face in organ transplant recipients, J. Cancer Res. Clin. Oncol., 2004, 130, 279–284; (c) G. Ekonjo, Y. Saleh, J. Kasiak, M. Gryboś, E. Teterycz, J. Korzeniewski, M. Siewiński, A. Dąbrowski and M. Słonina, In Vivo Application Of 5-Aminolevulinic Acid In The Treatment Of Papillomavirus Infection In Women With Cervical Lesions After Detection And Genotyping Using PCR Technique, Int. J. Gynecol. Obstet., 2005, 6(1), 9580.
R. L. S. Neris, C. M. Figueiredo, L. M. Higa, D. F. Araujo, C. A. M. Carvalho, B. R. F. Verçoza, M. O. L. Silva, F. A. Carneiro, A. Tanuri, A. M. O. Gomes, M. T. Bozza, A. T. Da Poian, C. Cruz-Oliveira and I. Assunção-Miranda, Co-protoporphyrin IX and Sn-protoporphyrin IX inactivate Zika, Chikungunya and other arboviruses by targeting the viral envelope, Sci. Rep., 2018, 8, 9805, DOI: 10.1038/s41598-018-27855-7.
I. Assunçao-Miranda, C. Cruz-Oliveira, R. L. S. Neris, C. M. Figueiredo, L. P. S. Pereira, D. Rodrigues, D. F. F. Araujo, A. T. Da Poian and M. T. Bozza, Inactivation of Dengue and Yellow Fever viruses by heme, cobalt-protoporphyrin IX and tin-protoporphyrin IX, J. Appl. Microbiol., 2016, 120, 790–804.
H. Yin, Y. Li, Z. Zou, W. Qiao, X. Yao, Y. Su and H. Guo, Inactivation of bovine immunodeficiency virus by photodynamic therapy with HMME, Chin. Opt. Lett., 2008, 6, 944–946.
H. Yin, Y. Li, Y. Zheng, X. Ye, L. Zheng, C. Li and Z. Xue, Photoinactivation of cell-free human immunodeficiency virus by hematoporphyrin monomethyl ether, Lasers Med. Sci., 2012, 27, 943–950.
M. C. Choi, S. G. Jung, H. Park, S. Y. Lee, C. Lee, Y. Y. Hwang and S. J. Kim, Photodynamic Therapy for Management of Cervical Intraepithelial Neoplasia II and III in Young Patients and Obstetric Outcomes, Lasers Surg. Med., 2013, 45, 564–572.
H. Ichimura, S. Yamaguchi, A. Kojima, T. Tanaka, K. Niiya, M. Takemori, K. Hasegawa and R. Nishimura, Eradication and reinfection of human papillomavirus after photodynamic therapy for cervical intraepithelial neoplasia, Int. J. Clin. Oncol., 2003, 8, 322–325.
D. Kessel and C. J. Dutton, Photodynamic Effects: Porphyrin vs Chlorin, Photochem. Photobiol., 1984, 40, 403–405.
A. A. Ryan and M. O. Senge, How green is green chemistry? Chlorophylls as a bioresource from biorefineries and their commercial potential in medicine and photovoltaics, Photochem. Photobiol. Sci., 2015, 14, 638–660.
A. Aggarwal, S. Thompson, S. Singh, B. Newton, A. Moore, R. Gao, X. Gu, S. Mukherjee and C. M. Drain, Photophysics of Glycosylated Derivatives of a Chlorin, Isobacteriochlorin and Bacteriochlorin for Photodynamic Theragnostics: Discovery of a Two-photon-absorbing Photosensitizer, Photochem. Photobiol., 2014, 90, 419–430; (b) S. Singh, A. Aggarwal, S. Thompson, J. P. C. Tome, X. C. Zhu, D. Samaroo, M. Vinodu, R. M. Gao and C. M. Drain, Synthesis and photophysical properties of thioglycosylatedchlorins, isobacteriochlorins and bacteriochlorins for bioimaging and diagnostics, Bioconjugate Chem., 2010, 21, 2136–2146; (c) R. Bonnett, R. D. White, U. J. Winfield and M. C. Berenbaum, Biochem. J., 1989, 261, 277–280.
A. Hajri, S. Wack, C. Meyer, M. K. Smith, C. Leberquier, M. Kedinger and M. Aprahamian, In Vitro and In Vivo Efficacy of Photofrin® and Pheophorbide a, a Bacteriochlorin, in Photodynamic Therapy of Colonic Cancer Cells, Photochem. Photobiol., 2002, 75, 140–148.
V. V. Zverev, O. V. Makarov, A. Z. Khashukoeva, O. A. Svitich, Y. E. Dobrokhotova, E. A. Markova, P. A. Labginov, S. A. Khlinova, E. A. Shulenina and L. V. Gankovskaya, In vitro studies of the antiherpetic effect of photodynamic therapy, Lasers Med. Sci., 2016, 31, 849–855.
L. Y. Wen, S.-M. Bae, J. H. Do, K.-S. Park and W. S. Ahn, The effects of photodynamic therapy with Photodithazine on HPV 16 E6/E7 associated cervical cancer model, J. Porphyrins Phthalocyanines, 2011, 15, 174–180.
M. L. Sagristá, F. Postigo, M. A. De Madariaga, R. M. Pintó, S. Caballero, A. Bosch, M. A. Vallés and M. Mora, Photodynamic inactivation of viruses by immobilized chlorin-containing liposomes, J. Porphyrins Phthalocyanines, 2009, 13, 578–588.
E. M. van der Snoek, J. C. den Hollander, J. B. Aans, H. J. C. M. Sterenborg, M. E. van der Ende and D. J. Robinson, Photodynamic Therapy With Systemic Meta-Tetrahydroxyphenylchlorin in the Treatment of Anal Intraepithelial Neoplasia, Grade 3, Lasers Surg. Med., 2012, 44, 637–644; (b) E. M. van der Snoek, A. Amelink, M. E. van der Ende, J. C. den Hollander, J. G. den Hollander, F. P. Kroon, R. Vriesendorp, H. A. M. Neumann and D. J. Robinson, Photodynamic Therapy With Topical Metatetrahydroxychlorin (Fosgel) Is Ineffective for the Treatment of Anal Intraepithelial Neoplasia, Grade III, J. Acquir. Immune Defic. Syndr., 2009, 52, 141–143; (c) S. M. Campbell and A. Crunow, Extensive vulval intraepithelial neoplasia treated with a new regime of systemic photodynamic therapy using meta-tetrahydroxychlorin (Foscan®), J. Eur. Acad. Dermatol. Venereol., 2008, 22, 502–503.
R. W. K. Wu, E. S. M. Chu, Z. Huang, C. S. Xu, C. W. Ip and C. M. N. Yow, FosPeg® PDT alters the EBV miRNAs and LMP1 protein expression in EBV positive nasopharyngeal carcinoma cells, J. Photochem. Photobiol., B, 2013, 127, 114–122.
B. Horowitz, B. Williams, S. Rywkin, A. M. Prince, D. Pascual, N. Geacintov and J. Valinsky, Inactivation of viruses in blood with aluminum phthalocyanine derivatives, Transfusion, 1991, 31, 102–108.
M.-R. Ke, J. M. Eastel, K. L. K. Ngai, Y.-Y. Cheung, P. K. S. Chan, M. Hui, D. K. P. Ng and P.-C. Lo, Photodynamic inactivation of bacteria and viruses using two monosubstituted zinc(II) phthalocyanines, Eur. J. Med. Chem., 2014, 84, 278–283; (b) M.-R. Ke, J. M. Eastel, K. L. K. Ngai, Y.-Y. Cheung, P. K. S. Chan, M. Hui, D. K. P. Ng and P.-C. Lo, Oligolysine-Conjugated Zinc(II) Phthalocyanines as Efficient Photosensitizers for Antimicrobial Photodynamic Therapy, Chem. – Asian J., 2014, 9, 1868–1875.
L. Sobotta, M. Wierzchowski, M. Mierzwicki, Z. Gdaniec, J. Mielcarek, L. Persoons, T. Goslinski and J. Balzarini, Photochemical studies and nanomolar photodynamic activities of phthalocyanines functionalized with 1,4,7-trioxanonyl moieties at their non-peripheral positions, J. Inorg. Biochem., 2016, 155, 76–81.
L. H. Z. Cocca, T. M. A. Oliveira, F. Gotardo, A. V. Teles, R. Menegatti, J. P. Siqueira, C. R. Mendonca, L. A. M. Bataus, A. O. Ribeiro, T. F. M. Souza, G. R. L. Souza, P. J. Goncalves and L. De Boni, Tetracarboxy-phthalocyanines: From excited state dynamics to photodynamic inactivation against Bovine herpesvirus type 1, J. Photochem. Photobiol., B, 2017, 175, 1–8.
M. Remichkova, L. Mukova, L. Nikolaeva-Glomb, N. Nikolova, L. Doumanova, V. Matareva, I. Angelov, V. Kussovski and A. S. Galabov, Virus inactivation under the photodynamic effect of phthalocyanine zinc(II) complexes, Z. Naturforsch., 2017, 72c, 123–128; (b) L. Nikolaeva-Glomb, L. Mukova, N. Nikolova, V. Kussovski, L. Doumanova, V. Mantareva, I. Angeloc, D. Wöhrle and A. S. Galabov, Photodynamic effect of some phthalocyanines on enveloped and naked viruses, Acta Virol., 2017, 61, 341–346.
S. Gaspard, C. Tempête and G. H. Werner, Studies on photoinactivation by various phthalocyanines of a free or replicating non-enveloped virus, J. Photochem. Photobiol., B, 1995, 31, 159–162.
Z. Smetana, E. Mendelson, J. Manor, J. E. van Lier, E. Ben-Hur, S. Salzburg and Z. Malik, Photodynamic inactivation of herpes viruses with phthalocyanine derivatives, J. Photochem. Photobiol., B, 1994, 22, 37–43; (b) C. M. Allen, J. M. Weber and J. E. van Lier, Sulfophthalocyanines for photodynamic inactivation of viruses in blood products: effect of structural modifications, Photochem. Photobiol., 1995, 62, 184–189.
A. N. Vzorov, J. Bozja, D. W. Dixon, L. G. Mazilli and R. W. Copmans, Parameters of inhibition of HIV-1 infection by small anionic microbicides, Antiviral Res., 2007, 73, 60–68.
K. O. Francois, C. Pannecouque, J. Auwerx, V. Lozano, M.-J. Pérez-Pérez, D. Schols and J. Balzarini, The Phthalocyanine Prototype Derivative Alcian Blue Is the First Synthetic Agent with Selective Anti-Human Immunodeficiency Virus Activity Due to Its gp120 Glycan-Binding Potential, Antimicrob. Agents Chemother., 2009, 53, 4852–4859.
A. Y. Tolbin, A. Y. Sukhorukov, S. L. Ioffe, O. A. Lobach, D. N. Nosik and L. G. Tomilova, Synthesis of a phthalocyanine-1,4,6,10-tetraazaadamantane conjugate and its activity against the human immunodeficiency virus, Mendeleev Commun., 2010, 20, 25–27.
A. R. Styczynski, K. N. Anwar, H. Sultana, A. Ghanem, N. Lurain, A. Chua, M. Ghassemi and R. M. Novak, In vitro antiretroviral activity and in vivo toxicity of the potential topical microbicide copper phthalocyanine sulfate, Virol. J., 2015, 12, 132–142.
E. Ben-Hur, M. M. Zuk, J. Oetjen, W.-S. Chan, L. Lenny and B. Horowitz, Photochemical Decontamination of Red Cell Concentrates with the Silicon Phthalocyanine Pc 4 and Red Light, J. Biomed. Opt., 1999, 4, 292–297.
A. C. E. Moor, A. E. Wagenaars-van Gompel, R. C. A. Hermanns, J. van der Meulen, J. Smit, J. Wilschut, A. Brand, T. M. A. R. Dubbelman and J. Van Steveninck, Inhibition of Various Steps in the Replication Cycle of Vesicular Stomatitis Virus Contributes to its Photoinactivation by AlPcS4 or Pc4 and Red Light, Photochem. Photobiol., 1999, 69, 353–359.
H. Margolis-Nunno, E. Ben-Hur, P. Gottlieb, R. Robinson, J. Oetjen and B. Horowitz, Inactivation by phthalocyanine photosensitization of multiple forms of human immunodeficiency virus in red cell concentrates, Transfusion, 1996, 36, 743–750.
E. Ben-Hur, J. Oetjen and B. Horowitz, Silicon phthalocyanine Pc 4 and red light causes apoptosis in HIV-infected cells, Photochem. Photobiol., 1997, 65, 456–460.
E. Ben-Hur, M. M. Zuk, M. E. Kenney, N. L. Oleinick, J. Mulvihill and B. Horowitz, Action spectra (660–700 nm) for virus inactivation and red cell damage photosensitized by the silicon phthalocyanine Pc 4, Laser Med. Sci., 1996, 11, 221–225.
E. Ben-Hur, N. E. Geacintov, B. Studamire, M. E. Kenney and B. Horowitz, The effect of irradiance on virus sterilization and photodynamic damage in red blood cells sensitized by phthalocyanines, Photochem. Photobiol., 1995, 61, 190–195; (b) E. Ben-Hur, S. Rywkin, I. Rosenthal, N. E. Geacintov and B. Horowitz, Virus inactivation in red cells concentrates by photosensitization with phthalocyanines: protection of red cells but not vesicular stomatitis virus with a water-soluble analogue of vitamin E, Transfusion, 1995, 35, 401–406.
A. C. Moor, J. W. Lagerberg, K. Tijssen, S. Foley, T. G. Truscott, I. E. Kochevar, A. Brand, T. M. A. R. Dubbelman and J. VanSteveninck, In vitro Fluence Rate Effects in Photodynamic Reactions with AlPcS4 as Sensitizer, Photochem. Photobiol., 1997, 66, 860–865.
I. B. Zavodnik, L. B. Zavodnik and M. J. Bryszewska, The mechanism of Zn-phthalocyanine photosensitized lysis of human erythrocytes, J. Photochem. Photobiol., B, 2002, 67, 1–10.
S. Rywkin, E. Ben-Hur, M. E. Reid, R. Oyen, H. Ralph and B. Horowitz, Selective protection against IgG binding to red cells treated with phthalocyanines and red light for virus inactivation, Transfusion, 1995, 35, 414–420.
F. H. E. Schagen, A. C. E. Moor, S. C. Cheong, S. J. Cramer, H. van Ormondt, A. J. van der Eb, T. M. A. R. Dubbelman and R. C. Hoeben, Photodynamic treatment of adenoviral vectors with visible light: an easy and convenient method for viral inactivation, Gene Ther., 1999, 6, 873–881.
H. Abe and S. J. Wagner, Analysis of viral DNA, protein and envelope damage after methylene blue, phthalocyanine derivative or merocyanine 540 photosensitization, Photochem. Photobiol., 1995, 61, 402–409.
M. E. Lim, Y. Zhang and J. J. H. Chu, Small NIR-to-VIS upconverting nanoparticles for photodynamic therapy, Proc. SPIE, 2012, 8232, 82320H.
N. A. Kuznetsova, O. A. Yuzhakova, A. E. Nedachin, R. A. Dmitrieva, T. V. Doskina, T. N. Maksimkina and O. L. Kaliya, Effect of support pore size on antiviral activity of the heterogeneous photosensitizer with phthalocyanine covalently linked to aminopropyl silica gel, J. Porphyrins Phthalocyanines, 2013, 17, 772–777.
J. L. Golbach, S. C. Ricke, C. A. O’Bryan and P. G. Crandall, Riboflavin in Nutrition, Food Processing, and Analysis - A Review, J. Food Res., 2014, 3, 23–35, DOI: 10.5539/jfr.v3n6p23; (b) K. Thakur, S. K. Tomar, A. K. Singh, S. Mandal and S. Arora, Riboflavin and health: A review of recent human research, Crit. Rev. Food Sci. Nutr., 2017, 57, 3650–3660.
A. Tsugita, Y. Okada and K. Uehara, Photosensitized inactivation of ribonucleic acids in the presence of riboflavin, Biochim. Biophys. Acta, 1965, 103, 360–363.
M. Wainwright, The emerging chemistry of blood product disinfection, Chem. Soc. Rev., 2002, 31, 128–136; (b) M. Wainwright, Pathogen Inactivation in Blood Products, Curr. Med. Chem., 2002, 9, 127–143.
M. Wainwright and M. S. Baptista, The application of photosensitisers to tropical pathogens in the blood supply, Photodiagn. Photodyn. Ther., 2011, 8, 240–248.
V. Salunkhe, P. F. van der Meer, D. de Korte, J. Seghatchian and L. Gutiérrez, Development of blood transfusion product pathogen reduction treatments: A review of methods, current applications and demands, Transfus. Apher. Sci., 2015, 52, 19–34.
V. J. Drew, L. Barro, J. Seghatchian and T. Burnouf, Towards pathogen inactivation of red blood cells and whole blood targeting viral DNA/RNA: design, technologies, and future prospects for developing countries, Blood Transfusion, 2017, 15, 512–521.
B. G. Solheim and J. Seghatchian, Update on pathogen reduction technology for therapeutic plasma: An overview, Transfus. Apher. Sci., 2006, 35, 83–90; (b) B. G. Solheim, Pathogen reduction of blood components, Transfus. Apher. Sci., 2008, 39, 75–82.
J. Seghatchian and G. de Sousa, Pathogen-reduction systems for blood components: The current position and future trends, Transfus. Apher. Sci., 2006, 35, 189–196.
J. Kaiser-Guignard, G. Canellini, N. Lion, M. Abonnenc, J.-C. Osselaer and J.-D. Tissot, The clinical and biological impact of new pathogen inactivation technologies on platelet concentrates, Blood Rev., 2014, 28, 235–241.
G. Rock, A comparison of methods of pathogen inactivation of FFP, Vox Sang., 2011, 100, 169–178.
R. Schuyler, Use of riboflavin for photoinactivation of pathogens in blood components, Transfus. Apher. Sci., 2001, 25, 189–190.
S. Marschner and R. Goodrich, Pathogen Reduction Technology Treatment of Platelets, Plasma and Whole Blood Using Riboflavin and UV Light, Transfus. Med. Hemother., 2011, 38, 8–18.
S. D. Keil, R. Bowen and S. Marschner, Inactivation of Middle East respiratory syndrome coronavirus (MERS-CoV) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment, Transfusion, 2016, 56, 2948–2952.
R. P. Goodrich, R. A. Edrich, J. Li and J. Seghatchian, The Mirasol™ PRT system for pathogen reduction of platelets and plasma: An overview of current status and future trends, Transfus. Apher. Sci., 2006, 35, 5–17.
H. L. Reddy, A. D. Dayan, J. Cavagnaro, S. Gad, J. Li and R. P. Goodrich, Toxicity Testing of a Novel Riboflavin-Based Technology for Pathogen Reduction and White Blood Cell Inactivation, Transfus. Med. Rev., 2008, 22, 133–153.
J. A. Cancelas, S. J. Slichter, N. Rugg, P. G. Pratt, S. Nestheide, J. Corson, E. Pellham, M. Huntington and R. P. Goodrich, Red blood cells derived from whole blood treated with riboflavin and ultraviolet light maintain adequate survival in vivo after 21 days of storage, Transfusion, 2017, 57, 1218–1225.
S. Yonemura, S. Doane, S. Keil, R. Goodrich, H. Pidcoke and M. Cardoso, Improving the safety of whole blood-derived transfusion products with a riboflavin-based pathogen reduction technology, Blood Transfusion, 2017, 15, 357–364.
L. Larrea, M. Calabuig, V. Roldán, J. Rivera, H.-M. Tsai, V. Vicente and R. Roig, The influence of riboflavin photochemistry on plasma coagulation factors, Transfus. Apher. Sci., 2009, 41, 199–204.
X. Liu, X. Zhao, X. Wang, J. Zhang, Y. Huang, Q. Mo, K. Qian and Y. Zhu, Photochemically Inactivated Hepatitis B Virus Promotes Upregulation of Th1-Type Cytokines, Photochem. Photobiol., 2012, 88, 1287–1292.
S. Marschner, L. D. Fast, W. M. Baldwin III, S. J. Slichter and R. P. Goodrich, White blood cell inactivation after treatment with riboflavin and ultraviolet light, Transfusion, 2010, 50, 2489–2498; (b) L. D. Fast, G. DiLeone and S. Marschner, Inactivation of human white blood cells in platelet products after pathogen reduction technology treatment in comparison to gamma irradiation, Transfusion, 2011, 51, 1397–1404.
A. A. Ignatova, O. V. Karpova, P. E. Trakhtman, S. A. Rumiantsev and M. A. Panteleev, Functional characteristics and clinical effectiveness of platelet concentrates treated with riboflavin and ultraviolet light in plasma and in platelet additive solution, Vox Sang., 2016, 110, 244–252.
M. Prudent, A. D’Alessandro, J.-P. Cazenave, D. V. Devine, C. Gachet, A. Greinacher, N. Lion, P. Schubert, L. Steil, T. Thiele, J.-D. Tissot, U. Völker and L. Zolla, Proteome Changes in Platelets After Pathogen Inactivation – An Interlaboratory Consensus, Transfus. Med. Rev., 2014, 28, 72–83.
V. Salunkhe, I. M. De Cuyper, P. Papadopoulos, P. F. van der Meer, B. B. Daal, M. Villa-Fajardo, D. de Korte, T. K. van den Berg and L. Gutiérrez, A comprehensive proteomics study on platelet concentrates: Platelet proteome, storage time and Mirasol pathogen reduction technology, Platelets, 2019, 30, 368–379.
L. Johnson and D. Marks, Treatment of Platelet Concentrates with the Mirasol Pathogen Inactivation System Modulates Platelet Oxidative Stress and NF-ΚB Activation, Transfus. Med. Hemother., 2015, 42, 167–173.
C. Silliman, Y. L. Fung, J. B. Ball and S. Y. Khan, Transfusion-related acute lung injury (TRALI): Current Concepts and Misconceptions, Blood Rev., 2009, 23, 245– 255.
B. Mallavia, N. Kwaan, S. Marschner, S. Yonemura and M. R. Looney, Mirasol pathogen reduction technology treatment of human whole blood does not induce acute lung injury in mice, PLoS One, 2017, 12, e0178725.
W. L. Fowlks, The mechanism of the photodynamic effect, J. Invest. Dermatol., 1959, 32, 233–247; (b) C. V. Hanson, J. L. Riggs and E. H. Lennette, Photochemical Inactivation of DNA and RNA Viruses by Psoralen Derivatives, J. Gen. Virol., 1978, 40, 345–358.
D. Bethea, B. Fullmer, S. Syed, G. Seltzer, J. Tiano, C. Rischko, L. Gillespie, D. Brown and F. P. Gasparro, Psoralen photobiology and photochemotherapy: 50 years of science and medicine, J. Dermatol. Sci., 1999, 19, 78–88.
J. Irsch, L. Pinkoski, L. Corash and L. Lin, INTERCEPT plasma: comparability with conventional fresh-frozen plasma based on coagulation function – an in vitro analysis, Vox Sang., 2010, 98, 47–55; (b) J. Irsch and L. Lin, Pathogen inactivation of platelet and plasma blood components for transfusion using the INTERCEPT blood system, Transfus. Med. Hemother., 2011, 38, 19–31.
A. D. Dayan, The Science of Safety: Toxicological Review of Amotosalen HCl, Transfus. Med. Hermother., 2004, 31(Suppl. 1), 17–23.
Description of the amotosalen-containing device at the FDA: Food and Drug Administration (2016), Intercept® Blood System for Platelets – Small Volume Processing Set (Pub. No. UCM427512), retrieved March 29, 2019 from https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/UCM427512.pdf.
V. Ciaravino, T. McCullough and A. D. Dayan, Pharmacokinetic and toxicology assessment of INTERCEPT (S-59 and UVA treated) platelets, Human Exp. Toxicol., 2001, 20, 533–550.
L. Lin, M. G. Conlan, J. Tessman, G. Cimino and S. Porter, Amotosalen interactions with platelet and plasma components: absence of neoantigen formation after photochemical treatment, Transfusion, 2005, 45, 1610–1620.
J. D. Roback, M. Conlan, W. L. Drew, P. Ljungman, W. G. Nichols and J. K. Preiksaitis, The Role of Photochemical Treatment With Amotosalen and UV-A Light in the Prevention of Transfusion-Transmitted Cytomegalovirus Infections, Transfus. Med. Rev., 2006, 20, 45–56; (b) L. Lin, C. V. Hanson, H. J. Alter, V. Jauvin, K. A. Bernard, K. K. Murthy, P. Metzel and L. Corash, Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light, Transfusion, 2005, 45, 580–590; (c) J.-P. Allain, J. Hsu, M. Pranmeth, D. Hanson, A. Stassinopoulos, L. Fischetti, L. Corash and L. Lin, Quantification of Viral Inactivation by Photochemical Treatment with Amotosalen and UVA Light, Using a Novel Polymerase Chain Reaction Inhibition Method with Preamplification, J. Infect. Dis., 2006, 194, 1737–1744; (d) F. Santa Maria, A. Laughhunn, M. C. Lanteri, M. Aubry, D. Musso and A. Stassinopoulos, Inactivation of Zika virus in platelet components using amotosalen and ultraviolet A illumination, Transfusion, 2017, 57, 2016– 2025.
K. Raviprakash, P. Sun, Y. Raviv, T. Luke, N. Martin and T. Kochel, Dengue virus photo-inactivated in presence of 1,5-iodonaphthylazide (INA) or AMT, a psoralen compound (4’-aminomethyl-trioxsalen) is highly immunogenic in mice, Human Vacc. Immunother., 2013, 9, 2336– 2341.
R. R. Tice, D. Gatehouse, D. Kirkland and G. Speit, The pathogen reduction treatment of platelets with S-59 HCl (Amotosalen) plus ultraviolet A light: Genotoxicity profile and hazard assessment, Mutation Res., 2007, 630, 50–68.
J.-L. H. Kerkhoffs, W. L. J. van Putten, V. M. J. Novotny, P. A. W. Te Boekhorst, M. R. Schipperus, J. J. Zwaginga, L. C. M. van Pampus, G. E. De Greef, M. Luten, P. C. Huijgens, A. Brand and D. J. van Rhenen, Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction, Br. J. Haematol., 2010, 150, 209–217.
M. Lozano, F. Knutson, R. Tardivel, J. Cid, R. M. Maymó, H. Löf, H. Roddie, J. Pelly, A. Docherty, C. Sherman, L. Lin, M. Propst, L. Corash and C. Prowse, A multi-centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet A pathogen inactivation stored for 6 or 7 d prior to transfusion, Br. J. Haematol., 2011, 153, 393–401.
M. Lozano and J. Cid, Analysis of reasons for not implementing pathogen inactivation for platelet concentrates, Transfus. Clin. Biol., 2013, 20, 158–164.
J. Cid, G. Escolar and M. Lozano, Therapeutic efficacy of platelet components treated with amotosalen and ultraviolet A pathogen inactivation method: results of a metaanalysis of randomized controlled trials, Vox Sang., 2012, 103, 322–330.
V. Bost, H. Odent-Malaure, P. Chavarin, H. Benamara, P. Fabrigli and O. Garraud, A regional haemovigilance retrospective study of four types of therapeutic plasma in a ten-year survey period in France, Vox Sang., 2013, 104, 337–341.
L. Corash and R. J. Benjamin, The role of hemovigilance and postmarketing studies when introducing innovation into transfusion medicine practice: the amotosalen-ultraviolet A pathogen reduction treatment model, Transfusion, 2016, 56, S29–S38; (b) S. Kleinman, W. Reed and A. Stassinopoulos, A patient-oriented risk–benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States, Transfusion, 2013, 53, 1603–1618; (c) see also: J.-P. Cazenave, Inactivation photochimique des pathogènes des plaquettes et du plasma: cinq ans d’utilisation clinique de routine et d’hémovigilance. Vers un changement de paradigme de la sécurité en transfusion, Transfus. Clin. Biol., 2011, 18, 53–61.
W. Nussbaumer, M. Amato, H. Schennach, M. Astl, C. Y. Chen, J.-S. Lin, L. Corash and R. J. Benjamin, Patient outcomes and amotosalen/UVA-treated platelet utilization in massively transfused patients, Vox Sang., 2017, 112, 249–256.
J. Cid, Prevention of transfusion-associated graft-versus-host disease with pathogen-reduced platelets with amotosalen and ultraviolet A light: a review, Vox Sang., 2017, 112, 607–613.
S. Stivala, S. Gobbato, L. Infanti, M. F. Reiner, N. Bonetti, S. C. Meyer, G. G. Camici, T. F. Lüscher, A. Buser and J. H. Beer, Amotosalen/ultraviolet A pathogen inactivation technology reduces platelet activatability, induces apoptosis and accelerates clearance, Haematologica, 2017, 102, 1650–1660.
T. Thiele, A. Sablewski, C. Iuga, T. Bakchoul, A. Bente, S. Görg, U. Völker, A. Greinacher and L. Steil, Profiling alterations in platelets induced by Amotosalen/UVA pathogen reduction and gamma irradiation – a LC-ESI-MS/MS-based proteomics approach, Blood Transfusion, 2012, 10(Suppl 2), s63–s70.
R. J. Benjamin, J. McCullough, P. D. Mintz, E. Snyder, W. D. Spotnitz, R. J. Rizzo, D. Wages, J.-S. Lin, L. Wood, L. Corash and M. G. Conlan, Therapeutic efficacy and safety of red blood cells treated with a chemical process (S-303) for pathogen inactivation: a Phase III clinical trial in cardiac surgery patients, Transfusion, 2005, 45, 1739– 1749.
A. Laughhunn, F. Santa Maria, J. Broult, M. C. Lanteri, A. Stassinopoulos, D. Musso and M. Aubry, Amustaline (S-303) treatment inactivates high levels of Zika virus in red blood cell components, Transfusion, 2017, 57, 779– 789; (b) J. A. Cancelas, J. L. Gottschall, N. Rugg, S. Graminske, M. A. Schott, A. North, N. Huang, N. Mufti, A. Erickson, S. Rico and L. Corash, Red blood cell concentrates treated with the amustaline (S-303) pathogen reduction system and stored for 35 days retain post-transfusion viability: results of a two-centre study, Vox Sang., 2017, 112, 210–218; (c) V. Brixner, A.-H. Kiessling, K. Madlener, M. M. Müller, J. Leibacher, S. Dombos, I. Weber, H.-U. Pfeiffer, C. Geisen, M. Schmidt, R. Henschler, A. North, N. Huang, N. Mufti, A. Erickson, C. Ernst, S. Rico, R. J. Benjamin, L. M. Corash and E. Seifried, Red blood cells treated with the amustaline (S-303) pathogen reduction system: a transfusion study in cardiac surgery, Transfusion, 2018, 58, 905–916.
P. Bonnafous, M.-C. Nicolaï, J.-C. Taveau, M. Chevalier, F. Barrière, J. Medina, O. Le Bihan, O. Adam, F. Ronzon and O. Lambert, Treatment of influenza virus with Beta-propiolactone alters viral membrane fusion, Biochim. Biophys. Acta, 2014, 1838, 355–363.
E. W. Schultz and A. P. Krueger, Inactivation of staphylococcus bacteriophage by methylene blue, Proc. Soc. Exp. Biol. Med., 1928, 26, 100–101.
F. Harris, L. K. Chatfield and D. A. Phoenix, Phenothiazinium Based Photosensitisers – Photodynamic Agents with a Multiplicity of Cellular Targets and Clinical Applications, Curr. Drug Targets, 2005, 6, 615–627.
M. Wainwright, Methylene blue derivatives – suitable photoantimicrobials for blood product disinfection?, Int. J. Antimicrob. Agents, 2000, 16, 381–394; (b) M. Wainwright, H. Mohr and W. H. Walker, Phenothiazinium derivatives for pathogen inactivation in blood products, J. Photochem. Photobiol., B, 2007, 86, 45– 58.
J. P. Tardivo, A. Del Giglio, C. S. de Oliveira, D. S. Gabrielli, H. C. Junqueira, D. B. Tada, D. Severino, R. de Fátima Turchiello and M. S. Baptista, Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications, Photodiagn. Photodyn. Ther., 2005, 2, 175–191.
G. Viola and F. Dall’Acqua, Photosensitization of Biomolecules by Phenothiazine Derivatives, Curr. Drug Targets, 2006, 7, 1135–1154; (b) R. A. Floyd, Serendipitous findings while researching oxygen free radicals, Free Radicals Biol. Med., 2009, 46, 1004–1013.
S. J. Wagner, A. Skripchenko, D. J. Donnelly, K. Ramaswamy and M. R. Detty, Chalcogenoxanthylium photosensitizers for the photodynamic purging of blood-borne viral and bacterial pathogens, Bioorg. Med. Chem., 2005, 13, 5927–5935; (b) S. J. Wagner, A. Skripchenko, D. Thompson-Montgomery, H. Awatefe, D. J. Donnelly and M. R. Detty, Use of a Red Cell Band 3-Ligand/ Antioxidant to Improve Red Cell Storage Properties Following Virucidal Phototeatment with Chalcogenoxanthylium Photosensitizers, Photochem. Photobiol., 2006, 82, 1595–1600.
P. K. Taylor and N. R. Doherty, Comparison of the treatment of herpes genitalis in men with proflavine photoinactivation, idoxuridine ointment, and normal saline, Br. J. Vener. Dis., 1975, 51, 125–129; (b) R. H. Kaufman, H. L. Gardner, D. Brown, C. Wallis, W. E. Rawls and J. L. Melnick, Herpes genitalis treated by photodynamic inactivation of virus, Am. J. Obstet. Gynecol., 1973, 117, 1144–1146; (c) T. D. Felber, E. B. Smith, J. M. Knox, C. Wallace and J. L. Melnick, Photodynamic Inactivation of Herpes Simplex. Report of a Clinical Trial, J. Am. Med. Assoc, 1973, 223, 289–292; (d) R. H. Kaufman, E. Adam, R. R. Mirkovic, J. L. Melnick and R. L. Young, Treatment of genital herpes simplex virus infection with photodynamic inactivation, Am. J. Obstet. Gynecol., 1978, 132, 861–869; (e) A. P. C. H. Roome, A. E. Tinkler, A. L. Hilton, D. G. Montefiore and D. Waller, Neutral red with photoinactivation in the treatment of herpes genitalis, Br. J. Vener. Dis., 1975, 51, 130–133; (f) J. Marotti, A. C. C. Aranha, C. D. P. Eduardo and M. S. Ribeiro, Photodynamic therapy can be effective as a treatment for herpes simplex labialis, Photomed. Laser Surg., 2009, 27, 357–363; (g) J. P. Tardivo, M. Wainwright and M. S. Baptista, Local clinical phototreatment of herpes infection in São Paulo, Photodiagn. Photodyn. Ther., 2012, 9, 118–121.
World Health Organization, Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products, WHO Tech. Rep. Ser., 2004, 924(Annex 4), 150–224.
F. F. Sperandio, J. Marotti, A. C. C. Aranha and C. de Paula Eduardo, Photodynamic therapy for the treatment of recurrent herpes labialis: Preliminary results, Gen. Dentistry, 2009, 57, 415–419; (b) J. Marotti, F. F. Sperandio, E. R. Fregnani, A. C. Correa-Aranha, P. M. de Freitas and C. d. P. Eduardo, High-Intensity Laser and Photodynamic Therapy as a Treatment for Recurrent Herpes Labialis, Photomed. Laser Surg., 2010, 28, 439–444; (c) K. M. Ramalho, R. G. Rocha, A. C. Correa-Aranha, S. R. de Barros Cunha, A. Simões, L. Campos and C. de P. Eduardo, Treatment of herpes simplex labialis in macule and vesicle phases with photodynamic therapy. Report of two cases, Photodiagn. Photodyn. Ther., 2015, 12, 321–323.
E. Steinmann, U. Gravemann, M. Friesland, J. Doerrbecker, T. H. Müller, T. Pietschmann and A. Seltsam, Two pathogen reduction technologies – methylene blue plus light and shortwave ultraviolet light – effectively inactivate hepatitis C virus in blood products, Transfusion, 2013, 53, 1010–1018; (b) J. J. Fryk, D. C. Marks, J. Hobson-Peters, D. Watterson, R. A. Hall, P. R. Young, S. Reichenberg, F. Tolksdorf, C. Sumian, U. Gravemann, A. Seltsam and H. M. Faddy, Reduction of Zika virus infectivity in platelet concentrates after treatment with ultraviolet C light and in plasma after treatment with methylene blue and visible light, Transfusion, 2017, 57, 2677–2682; (c) J. J. Fryk, D. C. Marks, J. Hobson-Peters, N. A. Prow, D. Watterson, R. A. Hall, P. R. Young, S. Reichenberg, C. Sumian and H. M. Faddy, Dengue and chikungunya viruses in plasma are effectively inactivated after treatment with methylene blue and visible light, Transfusion, 2016, 56, 2278–2285.
A. Elikaei, S. M. Hosseini, Z. Sharifi, H. Latifi, H. Nikbakht, H. Mirshafiee and A. Asadollahi, Methylene Blue Based Device for Pathogen Reduction in Human Plasma, Iran. J. Ped. Hematol. Oncol., 2013, 3, 97–102.
T.-W. Wong, H.-J. Huang, Y.-F. Wang, Y.-P. Lee, C.-C. Huang and C.-K. Yu, Methylene blue-mediated photodynamic inactivation as a novel disinfectant of enterovirus 71, J. Antimicrob. Chemother., 2010, 65, 2176– 2182.
L. M. Williamson, R. Cardigan and C. V. Prowse, Methylene blue-treated fresh-frozen plasma: what is its contribution to blood safety?, Transfusion, 2003, 43, 1322– 1329.
J. Seghatchian, W. H. Walker and S. Reichenberg, Updates on pathogen inactivation of plasma using Theraflex methylene blue system, Transfus. Apher. Sci., 2008, 38, 271–280; (b) J. Seghatchian, W. G. Struff and S. Reichenberg, Main Properties of the THERAFLEX MB-Plasma System for Pathogen Reduction, Transfus. Med. Hemother., 2011, 38, 55–64.
D. Crettaz, L. Sensebe, D.-H. Vu, P. Schneider, F. Depasse, W.-V. Bienvenut, M. Quadroni and J.-D. Tissot, Proteomics of methylene blue photo-treated plasma before and after removal of the dye by an absorbent filter, Proteomics, 2004, 4, 881–891.
C. Politis, L. Kavallierou, S. Hantziara, P. Katsea, V. Triantaphylou, C. Richardson, D. Tsoutsos, N. Anagnostopoulos, G. Gorgolidis and P. Ziroyannis, Quality and safety of fresh-frozen plasma inactivated and leucoreduced with the Theraflex methylene blue system including the Blueflex filter: 5 years’ experience, Vox Sang., 2007, 92, 319–326.
C. Politis, L. Kavallierou, S. Hantziara, M. Parara, E. Zervou, O. Katsarou, M. Hatzitaki, P. Fountouli, A. Gioka, K. Tzioura, S. Koumarianos, M. Asariotou and C. Richardson, Haemovigilance data on the use of methylene blue virally inactivated fresh frozen plasma with the Theraflex MB-Plasma System in comparison to quarantine plasma: 11 years’ experience, Transfus. Med., 2014, 24, 316–320.
R. Moog, S. Reichenberg, A. Hoburg and N. Müller, Quality of methylene blue–treated fresh-frozen plasma stored up to 27 months, Transfusion, 2010, 50, 516–518.
A. Rapaille, S. Reichenberg, T. Najdovski, N. Cellier, N. de Valensart and V. Deneys, Factor VIII and fibrinogen recovery in plasma after Theraflex methylene blue-treatment: effect of plasma source and treatment time, Blood Transfusion, 2014, 12, 226–231.
S. Reichenberg, U. Gravemann, C. Sumian and A. Seltsam, Challenge study of the pathogen reduction capacity of the THERAFLEX MB-Plasma technology, Vox Sang., 2015, 109, 129–137.
U. Gravemann, W. Handke, C. Sumian, I. Alvarez, S. Reichenberg, T. H. Müller and A. Seltsam, Plasma temperature during methylene blue/light treatment influences virus inactivation capacity and product quality, Vox Sang., 2018, 113, 368–377.
A. Pereira, Efficacy of different plasma sources in the treatment of thrombotic thrombocytopenic purpura, ISBT Sci. Ser., 2009, 4, 111–117.
P. Dewachter, S. Castro, P. Nicaise-Roland, S. Chollet-Martin, C. Le Beller, A. Lillo-le-Louet and C. Mouton-Faivre, Anaphylactic reaction after methylene blue-treated plasma transfusion, Br. J. Anaesth., 2011, 106, 687–689; (b) K. Nubret, M. Delhoume, I. Orsel, J. S. Laudy, M. Sellami and N. Nathan, Anaphylactic shock to fresh-frozen plasma inactivated with methylene blue, Transfusion, 2011, 51, 125–128; (c) P. M. Mertes, P. Demoly, A. Alperovitch, A. Bazin, J. Bienvenu, C. Caldani, B. Lamy, D. Laroche, M.-F. Leconte des Floris, J.-Y. Py, D. Rebibo, B. Willaert, C. Drouet, M. Carlier and A. Lienhart, Methylene blue–treated plasma: An increased allergy risk?, J. Allergy Clin. Immunol., 2012, 130, 808–812.
A. Seltsam and T. H. Mueller, Updated hemovigilance data do not show an increased risk of allergic reactions to methylene blue–treated plasma, J. Allergy Clin. Immunol., 2013, 131, 1253–1254.
H. New, H. Tinegate, L. Hunt, P. Bolton-Maggs and R. Cardigan, Joint UK BTS/HPA Professional Advisory Committee/Serious Hazards of Transfusion (SHOT) (2012), Position Statement Methylene Blue-Treated Plasma, 18 November 2012, retrieved March 29, 2019 from https: //www.transfusionguidelines.org/document-library/documents/position-statement-methylene-blue-treated-plasma/down-load-file/dl_ps_methylene_2012–11.pdf.
A. Seltsam and T. H. Müller, UVC Irradiation for Pathogen Reduction of Platelet Concentrates and Plasma, Transfus. Med. Hemother., 2011, 38, 43–54; (b) J. Seghatchian and F. Tolksdorf, Characteristics of the THERAFLEX UV-Platelets pathogen inactivation system – An update, Transfus. Apher. Sci., 2012, 46, 221–229.
T. Thiele, P. Pohler, T. Kohlmann, A. Sümnig, K. Aurich, K. Selleng, A. Westphal, T. Bakchoul, A. Petersmann, T. H. Müller, A. Greinacher and A. Seltsam, Tolerance of platelet concentrates treated with UVC-light only for pathogen reduction – a phase I clinical trial, Vox Sang., 2015, 109, 44–51.
V. Decraene, J. Pratten and M. Wilson, Cellulose Acetate Containing Toluidine Blue and Rose Bengal Is an Effective Antimicrobial Coating when Exposed to White Light, Appl. Environ. Microbiol., 2006, 72, 4436–4439.
A. Cossu, D. Ercan, R. V. Tikekar and N. Nitin, Antimicrobial Effect of Photosensitized Rose Bengal on Bacteria and Viruses in Model Wash Water, Food Bioprocess. Technol., 2016, 9, 441–451.
M. A. Namvar, M. Vahedi, H.-R. Abdolsamadi, A. Mirzaei, Y. Mohammadi and F. Azizi Jalilian, Effect of photodynamic therapy by 810 and 940 nm diode laser on Herpes Simplex Virus 1: An in vitro study, Photodiagn. Photodyn. Ther., 2019, 25, 87–91.
P. Gupta, A. Sharma, V. Mathias, Y. Raviv, R. Blumenthal and R. K. Maheshwari, Inactivation of non-enveloped virus by 1,5-iodonaphthyl azide, BMC Res. Notes, 2015, 8, 44, DOI: 10.1186/s13104-015-1006-2; (b) P. Gupta, A. Sharma, K. B. Spurgers, R. R. Bakken, L. T. Eccleston, J. W. Cohen, S. P. Honnold, P. J. Glass and R. K. Maheshwari, 1,5-Iodonaphthyl azide-inactivated V3526 protects against aerosol challenge with virulent venezuelan equine encephalitis virus, Vaccine, 2016, 34, 2762–2765.
J.-L. Sagripanti, H.-J. Marschall, L. Voss and B. H. Lseweh, Photochemical Inactivation of Alpha- and Poxviruses, Photochem. Photobiol., 2011, 87, 1369–1378.
S. Callaghan, M. A. Filatov, E. Sitte, H. Savoie, R. W. Boyle, K. J. Flanagan and M. O. Senge, Delayed release singlet oxygen sensitizers based on pyridone-appended porphyrins, Photochem. Photobiol. Sci., 2017, 16, 1371–1374.
A. Dewilde, C. Pellieux, S. Hajjam, P. Wattré, C. Pierlot, D. Hober and J.-M. Aubry, Virucidal activity of pure singlet oxygen generated by thermolysis of a water-soluble naphthalene endoperoxide, J. Photochem. Photobiol., B, 1996, 36, 23–29; (b) C. Pellieux, A. Dewilde, C. Pierlot and J.-M. Aubry, Bactericidal and virucidal activities of singlet oxygen generated by thermolysis of naphthalene endoperoxides, Methods Enzymol., 2000, 319, 197–207.
F. Käsermann and C. Kempf, Inactivation of enveloped viruses by singlet oxygen thermally generated from a polymeric naphthalene derivative, Antiviral Res., 1998, 38, 55–62.
M. C. Wolf, A. N. Freiberg, T. Zhang, Z. Akyol-Ataman, A. Grock, P. W. Hong, J. Lid, N. F. Watson, A. Q. Fang, H. C. Aguilar, M. Porotto, A. N. Honko, R. Damoiseaux, J. P. Miller, S. E. Woodson, S. Chantasirivisal, V. Fontanes, O. A. Negrete, P. Krogstad, A. Dasgupta, A. Moscona, L. E. Hensley, S. P. Whelan, K. F. Faull, M. R. Holbrook, M. E. Jung and B. Lee, A broad-spectrum antiviral targeting entry of enveloped viruses, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 3157–3162.
S. Montanaro, V. Lhiaubet-Vallet, M. Iesce, L. Previtera and M. A. Miranda, A mechanistic study on the phototoxicity of atorvastatin: Singlet oxygen generation by a phenanthrene-like photoproduct, Chem. Res. Toxicol., 2009, 22, 173–178.
N. Boens, V. Leen and W. Dehaen, Fluorescent indicators based on BODIPY, Chem. Soc. Rev., 2012, 41, 1130– 1172; (b) A. Loudet and K. Burgess, BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties, Chem. Rev., 2007, 107, 4891–4932.
C. S. Kue, S. Y. Ng, S. H. Voon, A. Kamkaew, L. Y. Chung, L. V. Kiewe and H. B. Lee, Recent strategies to improve boron dipyrromethene (BODIPY) for photodynamic cancer therapy: an updated review, Photochem. Photobiol. Sci., 2018, 17, 1691–1708.
B. L. Carpenter, X. Situ, F. Scholle, J. Bartelmess, W. W. Weare and R. A. Ghiladi, Antiviral, Antifungal and Antibacterial Activities of a BODIPY-Based Photosensitizer, Molecules, 2015, 20, 10604–10621.
S. Turner and C. Kaplan, Photoinactivation of Vaccinia Virus with Rose Bengal, J. Gen. Virol., 1968, 3, 433–443; (b) S. E. Brooks, V. Kaza, T. Nakamura and M. D. Trousdale, Photoinactivation of Herpes Simplex Virus by Rose Bengal and Fluorescein, Cornea, 1994, 13, 43–50.
A. Almeida, Â. Cunha, N. C. M. Gomes, E. Alves, L. Costa and M. A. F. Faustino, Phage Therapy and Photodynamic Therapy: Low Environmental Impact Approaches to Inactivate Microorganisms in Fish Farming Plants, Mar. Drugs, 2009, 7, 268–313; (b) P. Kanyal, R. Roshan P. M., M. Danish, A. Anita, A. Khati and R. S. Chauhan, Antimicrobial photodynamic therapy and its applicability in aquaculture systems and aquatic animal health management: An overview, J. Appl. Nat. Sci., 2016, 8, 506–514; (c) Z. Luksiene and L. Brovko, Antibacterial Photosensitization-Based Treatment for Food Safety, Food Eng. Rev., 2013, 5, 185–199; (d) D. Malara, C. Mielke, M. Oelgemöller, M. O. Senge and K. Heimann, Sustainable water treatment in aquaculture – photolysis and photodynamic therapy for the inactivation of Vibrio species, Aquacult. Res., 2017, 48, 2954–2962.
V. S. Ghate, W. Zhou and H.-G. Yuk, Perspectives and Trends in the Application of Photodynamic Inactivation for Microbiological Food Safety, Compr. Rev. Food Sci. Food Saf., 2019, 18, 402–424.
R. A. Craig, C. P. McCoy, S. P. Gorman and D. S. Jones, Photosensitisers – the progression from photodynamic therapy to anti-infective surfaces, Expert Opin. Drug Delivery, 2015, 12, 85–101; (b) L. Brovko, Photodynamic Treatment., A New Efficient Alternative for Surface Sanitation, Adv. Food Nutr. Res., 2010, 61, 119–147.
B. M. Pecson, L. Decrey and T. Kohn, Photoinactivation of virus on iron-oxide coated sand: Enhancing inactivation in sunlit waters, Water Res., 2012, 46, 1763–1770; (b) J. I. Nieto-Juarez and T. Kohn, Virus removal and inactivation by iron (hydr)oxide-mediated Fenton-like processes under sunlight and in the dark, Photochem. Photobiol. Sci., 2013, 12, 1596–1605.
W. Wang, J. C. Yu and P. K. Wong, Photocatalysts for Solar-Induced Water Disinfection: New Developments and Opportunities, Mater. Sci. Forum, 2013, 734, 63–89.
M. Shakeel, F. Jabeen, S. Shabbir, M. S. Asghar, M. S. Khan and A. S. Chaudhry, Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: a Review, Biol. Trace Elem. Res., 2016, 172, 1–36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiehe, A., O’Brien, J.M. & Senge, M.O. Trends and targets in antiviral phototherapy. Photochem Photobiol Sci 18, 2565–2612 (2019). https://doi.org/10.1039/c9pp00211a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00211a