Abstract
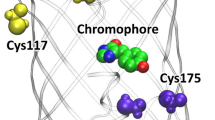
The fluorescence (FL) of calcium-discharged photoprotein (CaDP) can be altered by easily mutating CaDP without modifying coelenteramide (CLM), which is the decarboxylation product of coelenterazine in calcium-regulated photoprotein. The His22-Phe88-Trp92 triad (the ordering numbers of three amino acids are sorted by a crystal structure (PDB: 2F8P) of calcium-discharged obelin, i.e., CaDP-obelin) is closely related to CaDP-obelin FL, since it exists in close proximity to the 5-p-hydroxyphenyl of CLM. Therefore, it is important to thoroughly investigate how the mutations of this triad affect the emission color of CaDP-obelin FL. In this study, by mutating wild-type CaDP-obelin (WT) at the His22-Phe88-Trp92 triad, we theoretically constructed its nine mutants of separable FL colors. Through combined quantum mechanics and molecular mechanics (QM/MM) calculations and molecular dynamics (MD) simulations, the influence of the mutations of this triad on the CaDP-obelin FL was analyzed considering the H-bond effect and the charge effect. This study demonstrated that the mutations at the His22-Phe88-Trp92 triad redistribute the charges on the D–π-A molecule, CLM, change the charge transfer from the D to the (π + A>) moiety, and thereby alter the FL emission. Appending more negative charges on the phenolate moiety of CLM benefits the FL redshift.
Similar content being viewed by others
Notes and references
E. S. Vysotski and J. Lee, Ca2+-regulated photoproteins: structural insight into the bioluminescence mechanism, Acc. Chem. Res., 2004, 37, 405–415.
O. Shimomura, F. H. Johnson and Y. Saiga, Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea, J. Cell. Comp. Physiol., 1962, 59, 223–239.
O. Shimomura, F. H. Johnson and Y. Saiga, Extraction and properties of Halistaurin, a bioluminescent protein from the Hydromedusan Halistaura, J. Cell. Comp. Physiol., 1963, 62, 9–15.
L. D. Levine and W. W. Ward, Isolation and characterization of a photoprotein, “phialidin”, and a spectrally unique green-fluorescent protein from the bioluminescent jellyfish Phialidium gregarium, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1982, 72B, 77–85.
A. K. Campbell, Extraction, partial purification and properties of obelin, the calcium activated luminescent protein from the Hydroid Obelia geniculata, Biochem. J., 1974, 143, 411–418.
E. S. Vysotski, V. S. Bondar and V. N. Letunov, Extraction and purification of obelin, the Ca2+-dependent photoprotein from the hydroid Obelia longissima, Biokhimiya, 1989, 54, 965–973.
M. Anctil and O. Shimomura, Mechanism of photoinactivation and re-activation in the bioluminescence system of the ctenophore Mnemiopsis, Biochem. J., 1984, 221, 269–272.
J. G. Morin and J. W. Hastings, Biochemistry of the bioluminescence of colonial hydroids and other coelenterates, J. Cell. Physiol., 1971, 77, 305–312.
W. W. Ward and H. H. Seliger, Extraction and purification of calcium-activated photoproteins from the ctenophores Mnemiopsis sp. and Beroe ovata, Biochemistry, 1974, 13, 1491–1499.
M. L. Powers, A. G. McDermott, N. C. Shaner and S. H. Haddock, Expression and characterization of the calcium-activated photoprotein from the ctenophore Bathocyroe fosteri: insights into light-sensitive photoproteins, Biochem. Biophys. Res. Commun., 2013, 431, 360–366.
L. P. Burakova, S. V. Markova, S. Golz, L. A. Frank and E. S. Vysotski, The isospecies of Ca2+-regulated photoprotein bolinopsin from Bolinopsis infundibulum, Luminescence, 2006, 21, 273–273.
J. F. Head, S. Inouye, K. Teranishi and O. Shimomura, The crystal structure of the photoprotein aequorin at 2.3 Å resolution, Nature, 2000, 405, 372–376.
Z.-J. Liu, E. S. Vysotski, L. Deng, J. Lee, J. Rose and B.-C. Wang, Atomic resolution structure of obelin: soaking with calcium enhances electron density of the second oxygen atom substituted at the C2-position of coelenterazine, Biochem. Biophys. Res. Commun., 2003, 311, 433–439.
L. P. Burakova, P. V. Natashin, S. V. Markova, E. V. Eremeeva, N. P. Malikova, C. Cheng, Z. J. Liu and E. S. Vysotski, Mitrocomin from the jellyfish Mitrocoma cellularia with deleted C-terminal tyrosine reveals a higher bioluminescence activity compared to wild type photoprotein, J. Photochem. Photobiol., B, 2016, 162, 286–297.
M. S. Titushin, Y. Feng, G. A. Stepanyuk, Y. Li, S. V. Markova, S. Golz, B. C. Wang, J. Lee, J. Wang, E. S. Vysotski and Z. J. Liu, NMR-derived topology of a GFP-photoprotein energy transfer complex, J. Biol. Chem., 2010, 285, 40891–40900.
L. P. Burakova, G. A. Stepanyuk, E. V. Eremeeva and E. S. Vysotski, Role of certain amino acid residues of the coelenterazine-binding cavity in bioluminescence of lightsensitive Ca2+-regulated photoprotein berovin, Photochem. Photobiol. Sci., 2016, 15, 691–704.
E. S. Vysotski, S. V. Markova and L. A. Frank, Calcium-regulated photoproteins of marine coelenterates, Mol. Biol., 2006, 40, 355–367.
Y. Zhao, Y. Shi, W. Zhao, X. Huang, D. Wang, N. Brown, J. Brand and J. Zhao, CcbP, a calcium-binding protein from Anabaena sp. PCC 7120, provides evidence that calcium ions regulate heterocyst differentiation, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 5744–5748.
S. Inouye, Blue fluorescent protein from the calcium-sensitive photoprotein aequorin is a heat resistant enzyme, catalyzing the oxidation of coelenterazine, FEBS Lett., 2004, 577, 105–110.
L. A. Frank, Recombinant hybrid proteins as biospecific reporters for bioluminescent microassay, J. Sib. Fed. Univ. Biol., 2018, 11, 166–173.
A. Bakayan, B. Domingo, C. F. Vaquero, N. Peyrieras and J. Llopis, Fluorescent protein-photoprotein fusions and their applications in calcium imaging, Photochem. Photobiol., 2017, 93, 448–465.
S. Inouye and Y. Sahara-Miura, A fusion protein of the synthetic IgG-binding domain and aequorin: Expression and purification from, E. coli cells and its application, Protein Expression Purif., 2017, 137, 58–63.
S. Sharifian, A. Homaei, R. Hemmati, R. B. Luwor and K. Khajeh, The emerging use of bioluminescence in medical research, Biomed. Pharmacother., 2018, 101, 74–86.
V. V. Krasitskaya, A. N. Kudryavtsev, O. Shimomura and L. A. Frank, Obelin mutants as reporters in bioluminescent dual-analyte binding assay, Anal. Methods, 2013, 5, 636–640.
G. V. Aglyamova, M. E. Hunt, C. K. Modi and M. V. Matz, Multi-colored homologs of the green fluorescent protein from hydromedusa Obelia sp, Photochem. Photobiol. Sci., 2011, 10, 1303–1309.
L. A. Frank, V. V. Borisova, S. V. Markova, N. P. Malikova, G. A. Stepanyuk and E. S. Vysotski, Violet and greenish photoprotein obelin mutants for reporter applications in dual-color assay, Anal. Bioanal. Chem., 2008, 391, 2891–2896.
R. R. Alieva, N. V. Belogurova, A. S. Petrova and N. S. Kudryasheva, Fluorescence properties of Ca2+-independent discharged obelin and its application prospects, Anal. Bioanal. Chem., 2013, 405, 3351–3358.
N. Komatsu, K. Terai, A. Imanishi, Y. Kamioka, K. Sumiyama, T. Jin, Y. Okada, T. Nagai and M. Matsuda, A platform of BRET-FRET hybrid biosensors for optogenetics, chemical screening, and in vivo imaging, Sci. Rep., 2018, 8, 8984.
Y. Nakajima, T. Kimura, K. Sugata, T. Enomoto, A. Asakawa, H. Kubota, M. Ikeda and Y. Ohmiya, Multicolor luciferase assay system: One-step monitoring of multiple gene expressions with a single substrate, BioTechniques, 2005, 38, 891–894.
R. Ogura, N. Matsuo, N. Wako, T. Tanaka, S. Ono and K. Hiratsuka, Multi-color luciferases as reporters for monitoring transient gene expression in higher plants, Plant Biotechnol., 2005, 22, 151–155.
C. Wu, C. Suzuki-Ogoh and Y. Ohmiya, Dual-reporter assay using two secreted luciferase genes, BioTechniques, 2007, 42, 290–292.
Y. Nakajima and Y. Ohmiya, Bioluminescence assays: Multicolor luciferase assay, secreted luciferase assay and imaging luciferase assay, Expert Opin. Drug Discovery, 2010, 5, 835–849.
R. R. Alieva, N. V. Belogurova, A. S. Petrova and N. S. Kudryasheva, Effects of alcohols on fluorescence intensity and color of a discharged-obelin-based biomarker, Anal. Bioanal. Chem., 2014, 406, 2965–2974.
B. van Oort, E. V. Eremeeva, R. B. M. Koehorst, S. P. Laptenok, H. van Amerongen, W. J. H. van Berkel, N. P. Malikova, S. V. Markova, E. S. Vysotski, A. J. W. J. Visser and J. Lee, Picosecond fluorescence relaxation spectroscopy of the calcium-discharged photoproteins aequorin and obelin, Biochemistry, 2009, 48, 10486–10491.
L. Deng, S. V. Markova, E. S. Vysotski, Z. J. Liu, J. Lee, J. Rose and B. C. Wang, Preparation and X-ray crystallographica analysis of the Ca2+-discharged photoprotein obelin, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2004, 60, 512–514.
A. Petrova, N. Belogurova, R. Alieva and N. Kudryasheva, Effect of DMSO on fluorescence properties of Ca2+-discharged photoprotein obelin, Luminescence, 2014, 29, 40–40.
O. Shimomura and F. H. Johnson, Calcium binding, quantum yield, and emitting molecule in aequorin bioluminescence, Nature, 1970, 227, 1356–1357.
O. Shimomura and F. H. Johnson, Regeneration of the photoprotein aequorin, Nature, 1975, 256, 236–238.
N. V. Belogurova and N. S. Kudryasheva, Discharged photoprotein obelin: fluorescence peculiarities, J. Photochem. Photobiol., B, 2010, 101, 103–108.
Z.-J. Liu, G. A. Stepanyuk, E. S. Vysotski, J. Lee, S. V. Markova, N. P. Malikova and B.-C. Wang, Crystal structure of obelin after Ca2+-triggered bioluminescence suggests neutral coelenteramide as the primary excited state, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 2570–2575.
E. V. Eremeeva and E. S. Vysotski, Exploring bioluminescence function of the Ca2+-regulated photoproteins with site-directed mutagenesis, Photochem. Photobiol., 2019, 95, 8–23.
E. V. Eremeeva, S. V. Markova, L. A. Frank, A. J. Visser, W. J. van Berkel and E. S. Vysotski, Bioluminescent and spectroscopic properties of His-Trp-Tyr triad mutants of obelin and aequorin, Photochem. Photobiol. Sci., 2013, 12, 1016–1024.
Z.-J. Liu, E. S. Vysotski, C.-J. Chen, J. P. Rose, J. Lee and B.-C. Wang, Structure of the Ca2+-regulated photoprotein obelin at 1.7 angstrom resolution determined directly from its sulfur substructure, Protein Sci., 2000, 9, 2085–2093.
L. Yue, QM/MM investigations on the bioluminescent decomposition of coelenterazine dioxetanone in Obelin, Chem. Res. Chin. Univ., 2018, 34, 758–766.
L. Yue, Y.-J. Liu and W.-H. Fang, Mechanistic insight into the chemiluminescent decomposition of firefly dioxetanone, J. Am. Chem. Soc., 2012, 134, 11632–11639.
B.-W. Ding and Y.-J. Liu, Bioluminescence of firefly squid via mechanism of single Electron-transfer oxygenation and Charge-transfer-induced luminescence, J. Am. Chem. Soc., 2017, 139, 1106–1119.
N. P. Malikova, G. A. Stepanyuk, L. A. Frank, S. V. Markova, E. S. Vysotski and J. Lee, Spectral tuning of obelin bioluminescence by mutations of Trp92, FEBS Lett., 2003, 554, 184–188.
G. A. Stepanyuk, S. Golz, S. V. Markova, L. A. Frank, J. Lee and E. S. Vysotski, Interchange of aequorin and obelin bioluminescence color is determined by substitution of one active site residue of each photoprotein, FEBS Lett., 2005, 579, 1008–1014.
S. V. Markova, E. S. Vysotski, J. R. Blinks, L. P. Burakova, B. C. Wang and J. Lee, Obelin from the bioluminescent marine Hydroid Obelia geniculata: Cloning, Expression, and comparison of some properties with those of other Ca2+-regulated photoproteins, Biochemistry, 2002, 41, 2227–2236.
N. V. Belogurova, N. S. Kudryasheva, R. R. Alieva and A. G. Sizykh, Spectral components of bioluminescence of aequorin and obelin, J. Photochem. Photobiol., B, 2008, 92, 117–122.
S. Chen, I. Navizet, R. Lindh, Y. Liu and N. Ferré, Hybrid QM/MM simulations of the obelin bioluminescence and fluorescence reveal an unexpected light emitter, J. Phys. Chem. B, 2014, 118, 2896–2903.
S.-F. Chen, N. Ferré and Y.-J. Liu, QM/MM study on the light emitters of aequorin chemiluminescence, bioluminescence, and fluorescence: a general understanding of the bioluminescence of several marine organisms, Chem. –, Eur. J., 2013, 19, 8466–8472.
R. R. Alieva, F. N. Tomilin, A. A. Kuzubov, S. G. Ovchinnikov and N. S. Kudryasheva, Ultraviolet fluorescence of coelenteramide and coelenteramide-containing fluorescent proteins. Experimental and theoretical study, J. Photochem. Photobiol., B, 2016, 162, 318–323.
P. V. Natashin, S. V. Markova, J. Lee, E. S. Vysotski and Z.-J. Liu, Crystal structures of the F88Y obelin mutant before and after bioluminescence provide molecular insight into spectral tuning among hydromedusan photoproteins, FEBS J., 2014, 281, 1432–1445.
Z. S. Li, L. Y. Zou, C. G. Min and A. M. Ren, The effect of micro-environment on luminescence of aequorin: the role of amino acids and explicit water molecules on spectroscopic properties of coelenteramide, J. Photochem. Photobiol., B, 2013, 127, 94–99.
K. Mori, S. Maki, H. Niwa, H. Ikeda and T. Hirano, Real light emitter in the bioluminescence of the calcium-activated photoproteins aequorin and obelin: light emission from the singlet-excited state of coelenteramide phenolate anion in a contact ion pair, Tetrahedron, 2006, 62, 6272–6288.
E. S. Vysotski, Z. J. Liu, S. V. Markova, J. R. Blinks, L. Deng, L. A. Frank, M. Herko, N. P. Malikova, J. P. Rose, B. C. Wang and J. Lee, Violet bioluminescence and fast kinetics from W92F obelin: Structure-based proposals for the bioluminescence triggering and the identification of the emitting species, Biochemistry, 2003, 42, 6013–6024.
O. Shimomura and K. Teranishi, Light-emitters involved in the luminescence of coelenterazine, Luminescence, 2000, 15, 51–58.
L. Deng, E. S. Vysotski, Z. J. Liu, S. V. Markova, N. P. Malikova, J. Lee, J. Rose and B. C. Wang, Structural basis for the emission of violet bioluminescence from a W92F obelin mutant, FEBS Lett., 2001, 506, 281–285.
E. Runge and E. K. U. Gross, Density-functional theory for time-dependent systems, Phys. Rev. Lett., 1984, 52, 997–1000.
T. Yanai, D. P. Tew and N. C. Handy, A new hybrid exchange–correlation functional using the Coulombattenuating method (CAM-B3LYP), Chem. Phys. Lett., 2004, 393, 51–57.
P. C. Hariharan and J. A. Pople, The influence of polarization functions on molecular orbital hydrogenation energies, Theor. Chim. Acta, 1973, 28, 213–222.
W. J. Hehre, R. Ditchfield and J. A. Pople, Self—consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules, J. Chem. Phys., 1972, 56, 2257–2261.
J. M. Wang, P. Cieplak and P. A. Kollman, How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules?, J. Comput. Chem., 2000, 21, 1049–1074.
A. E. Reed, R. B. Weinstock and F. Weinhold, Natural population analysis, J. Chem. Phys., 1985, 83, 735–746.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. Peralta, J. E. Xxx, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009.
J. W. Ponder, Tinker-SoftwareTools for Molecular Design, Version 6.3, Washington University School of Medicine, St. Louis, MO(USA), 2014. The most updated version for the Tinker program can be obtained from the Internet at: http://dasher.wustl.edu/tinker.
N. Ferré and J. G. Ángyán, Approximate electrostatic interaction operator for QM/MM calculations, Chem. Phys. Lett., 2002, 356, 331–339.
F. Melaccio, M. Olivucci, R. Lindh and N. Ferré, Unique QM/MM potential energy surface exploration using microiterations, Int. J. Quantum Chem., 2011, 111, 3339–3346.
F. Collette, T. Renger, F. Müh and M. Schmidt am Busch, Red/Green color tuning of visual rhodopsins: Electrostatic theory provides a quantitative explanation, J. Phys. Chem. B, 2018, 122, 4828–4837.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9pp00191c
Rights and permissions
About this article
Cite this article
Gao, M., Ding, BW. & Liu, YJ. Tuning the fluorescence of calcium-discharged photoprotein obelin via mutating at the His22-Phe88-Trp92 triad – a QM/MM study†. Photochem Photobiol Sci 18, 1823–1832 (2019). https://doi.org/10.1039/c9pp00191c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00191c