Abstract
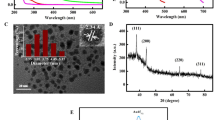
Biomolecules like cysteine and cytosine play a significant role in many physiological processes, and their unusual level in biological systems can lead to many diseases including cancer. Indeed, the need for selective detection of these moieties by a fluorescence probe is imperative. Thus, thiophene based Schiff N,N′-bis(thiophene-2-ylmethylene)thiophenemethane (BMTM) was synthesized and then characterized using several analytical techniques before converting it into organic nanoparticles (ONPs). Then, fluorescent organic inorganic nanohybrids (FONs) were obtained after decorating ONPs with AuNPs to yield BMTM-Au-ONPs (FONPs). The morphology of the particles, analyzed using a Transmission Electron Microscope (TEM), shows that AuNPs were embedded with low density organic matter (ONPs). FONPs were employed to recognize cysteine and cytosine simultaneously. No interference was observed from other moieties such as guanine, uracyl, NADH, NAD, ATP, and adenine during the detection. It means that the intensity of the fluorescence signal was significantly changed (enhanced for cytosine and quenched for cysteine). So, FONPs were used to detect cysteine and cytosine in real samples, like Saccharomyces cerevisiae cells. As expected, no considerable fluorescence signal for cysteine was observed, while for cytosine, strong fluorescence signals were detected in the cells. DFT was used to explain the interaction of FONPs with cysteine or cytosine.
Similar content being viewed by others
Notes and references
A. J. Zucchero, P. L. McGrier and U. H. F. Bunz, Cross-Conjugated Cruciform Fluorophores, Acc. Chem. Res., 2010, 43, 397–408.
J. Gao, H. M. Wang, L. Wang, J. Y. Wang, D. L. Kong and Z. M. Yang, Enzyme Promotes the Hydrogelation from a Hydrophobic Small Molecule, J. Am. Chem. Soc., 2009, 131, 11286–11287.
A. L. Balch, Dynamic Crystals: Visually Detected Mechanochemical Changes in the Luminescence of Gold and Other Transition-Metal Complexes, Angew. Chem., Int. Ed., 2009, 48, 2641–2644.
A. Chowdhury, P. Howlader and P. S. Mukherjee, Mechanofluorochromic Pt-II Luminogen and Its Cysteine Recognition, Chem. –, Eur. J., 2016, 22, 1424–1434.
A. Saini, J. Singh, R. Kaur, N. Singh and N. Kaur, Naphthalimide-based organic nanoparticles for aluminium recognition in acidic soil and aqueous media, New J. Chem., 2014, 38, 4580–4586.
H. E. Toma, Supramolecular nanotechnology: from molecules to devices, Curr. Sci., 2008, 95, 1202–1225.
G. L. Hornyak G., H. Tibbals and A. Rao, Introduction to Nanoscience, 2008.
R. Narayanan and M. A. El-Sayed, Catalysis with Transition Metal Nanoparticles in Colloidal Solution: Nanoparticle Shape Dependence and Stability, J. Phys. Chem. B, 2005, 109, 12663–12676.
D. A. Giljohann, D. S. Seferos, P. C. Patel, J. E. Millstone, N. L. Rosi and C. A. Mirkin, Oligonucleotide loading deter-mines cellular uptake of DNA-modified gold nanoparticles, Nano Lett., 2007, 7, 3818–3821.
D. S. Seferos, A. E. Prigodich, D. A. Giljohann, P. C. Patel and C. A. Mirkin, Polyvalent DNA Nanoparticle Conjugates Stabilize Nucleic Acids, Nano Lett., 2009, 9, 308–311.
A. E. Prigodich, P. S. Randeria, W. E. Briley, N. J. Kim, W. L. Daniel, D. A. Giljohann and C. A. Mirkin, Multiplexed Nanoflares: mRNA Detection in Live Cells, Anal. Chem., 2012, 84, 2062–2066.
Y. Zhang, M. Li, H. Y. Liu, S. G. Ge and J. H. Yu, Label-free colorimetric logic gates based on free gold nanoparticles and the coordination strategy between cytosine and silver ions, New J. Chem., 2016, 40, 5516–5522.
H. Y. Niu, S. H. Wang, Z. Zhou, Y. R. Ma, X. F. Ma and Y. Q. Cai, Sensitive Colorimetric Visualization of Perfluorinated Compounds Using Poly(ethylene glycol) and Perfluorinated Thiols Modified Gold Nanoparticles, Anal. Chem., 2014, 86, 4170–4177.
Z. Z. Huang, H. N. Wang and W. S. Yang, Glutathione-facilitated design and fabrication of gold nanoparticle-based logic gates and keypad lock, Nanoscale, 2014, 6, 8300–8305.
Z. Q. Gao, K. C. Deng, X. D. Wang, M. Miro and D. P. Tang, High-Resolution Colorimetric Assay for Rapid Visual Readout of Phosphatase Activity Based on Gold/Silver Core/Shell Nanorod, ACS Appl. Mater. Interfaces, 2014, 6, 18243–18250.
L. Jiang, Y. H. Sun, C. Nowak, A. Kibrom, C. J. Zou, J. Ma, H. Fuchs, S. Z. Li, L. F. Chi and X. D. Chen, Patterning of Plasmonic Nanoparticles into Multiplexed One-Dimensional Arrays Based on Spatially Modulated Electrostatic Potential, ACS Nano, 2011, 5, 8288–8294.
H. Jans and Q. Huo, Gold nanoparticle-enabled biological and chemical detection and analysis, Chem. Soc. Rev., 2012, 41, 2849–2866.
J. H. Lee, M. V. Yigit, D. Mazumdar and Y. Lu, Molecular diagnostic and drug delivery agents based on aptamernanomaterial conjugates, Adv. Drug Delivery Rev., 2010, 62, 592–605.
C. W. Corti, R. J. Holliday and D. T. Thompson, Developing new industrial applications for gold: Gold nanotechnology, Gold Bull., 2002, 35, 111–136.
M. Beytur, F. Kardas, O. Akyildirim, A. Ozkan, B. Bankoglu, H. Yuksek, M. L. Yola and N. Atar, A highly selective and sensitive voltammetric sensor with molecularly imprinted polymer based silver@gold nanoparticles/ionic liquid modified glassy carbon electrode for determination of ceftizoxime, J. Mol. Liq., 2018, 251, 212–217.
M. L. Yola and N. Atar, A novel voltammetric sensor based on gold nanoparticles involved in p-aminothiophenol functionalized multi-walled carbon nanotubes: Application to the simultaneous determination of quercetin and rutin, Electrochim. Acta, 2014, 119, 24–31.
M. L. Yola, N. Atar, Z. Ustundag and A. O. Solak, A novel voltammetric sensor based on p-aminothiophenol functionalized graphene oxide/gold nanoparticles for determining quercetin in the presence of ascorbic acid, J. Electroanal. Chem., 2013, 698, 9–16.
V. K. Gupta, M. L. Yola, M. S. Qureshi, A. O. Solak, N. Atar and Z. Ustundag, A novel impedimetric biosensor based on graphene oxide/gold nanoplatform for detection of DNA arrays, Sens. Actuators, B, 2013, 188, 1201–1211.
M. L. Yola, T. Eren and N. Atar, A sensitive molecular imprinted electrochemical sensor based on gold nanoparticles decorated graphene oxide: Application to selective determination of tyrosine in milk, Sens. Actuators, B, 2015, 210, 149–157.
M. L. Yola, N. Atar, T. Eren, H. Karimi-Maleh and S. B. Wang, Sensitive and selective determination of aqueous triclosan based on gold nanoparticles on polyoxometalate/reduced graphene oxide nanohybrid, RSC Adv., 2015, 5, 65953–65962.
N. Atar, T. Eren and M. L. Yola, Ultrahigh capacity anode material for lithium ion battery based on rod gold nanoparticles decorated reduced graphene oxide, Thin Solid Films, 2015, 590, 156–162.
N. Atar, T. Eren, M. L. Yola, H. Karimi-Maleh and B. Demirdogen, Magnetic iron oxide and iron oxide@gold nanoparticle anchored nitrogen and sulfur-functionalized reduced graphene oxide electrocatalyst for methanol oxidation, RSC Adv., 2015, 5, 26402–26409.
M. C. Daniel and D. Astruc, Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology, Chem. Rev., 2004, 104, 293–346.
X. J. Liu, D. L. Yang, W. Q. Chen, L. Yang, F. P. Qi and X. Z. Song, A red-emitting fluorescent probe for specific detection of cysteine over homocysteine and glutathione with a large Stokes shift, Sens. Actuators, B, 2016, 234, 27–33.
Y. Suzuki, K. Suda, Y. Matsuyama, S. Era and A. Soejima, Close relationship between redox state of human serum albumin and serum cysteine levels in non-diabetic CKD patients with various degrees of renal function, Clin. Nephrol., 2014, 82, 320–325.
S. Y. Zhang, C. N. Ong and H. M. Shen, Involvement of proapoptotic Bcl-2 family members in parthenolideinduced mitochondrial dysfunction and apoptosis, Cancer Lett., 2004, 211, 175–188.
C. A. Huerta-Aguilar, P. Thangarasu and J. G. Mora, Structural influence in the interaction of cysteine with five coordinated copper complexes: Theoretical and experimental studies, J. Mol. Struct., 2018, 1157, 660–671.
S. C. Lu, Regulation of glutathione synthesis, Mol. Aspects Med., 2009, 30, 42–59.
H. Tapiero, D. M. Townsend and K. D. Tew, The antioxidant role of selenium and seleno-compounds, Biomed. Pharmacother., 2003, 57, 134–144.
S. Shahrokhian, Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode, Anal. Chem., 2001, 73, 5972–5978.
S. K. Kim, D. H. Lee, J. I. Hong and J. Yoon, Chemosensors for Pyrophosphate, Acc. Chem. Res., 2009, 42, 23–31.
A. T. Wright and E. V. Anslyn, Differential receptor arrays and assays for solution-based molecular recognition, Chem. Soc. Rev., 2006, 35, 14–28.
H. Refsum, A. D. Smith, P. M. Ueland, E. Nexo, R. Clarke, J. McPartlin, C. Johnston, F. Engbaek, J. Schneede, C. McPartlin and J. M. Scott, Facts and recommendations about total homocysteine determinations: An expert opinion, Clin. Chem., 2004, 50, 3–32.
J. B. J. van Meurs, R. A. M. Dhonukshe-Rutten, S. M. F. Pluijm, M. van der Klift, R. de Jonge, J. Lindemans, L. de Groot, A. Hofman, J. C. M. Witteman, J. van Leeuwen, M. M. B. Breteler, P. Lips, H. A. P. Pols and A. G. Uitterlinden, Homocysteine levels and the risk of osteoporotic fracture, N. Engl. J. Med., 2004, 350, 2033–2041.
J. B. J. van Meurs, H. A. P. Pols and A. G. Uitterlinden, Homocysteine as a predictive factor for hip fracture in older persons - Reply, N. Engl. J. Med., 2004, 351, 1029–1029.
A. C. Flint, H. Kamel, B. B. Navi, V. A. Rao, B. S. Faigeles, C. Conell, J. G. Klingman, N. K. Hills, M. Nguyen-Huynh, S. P. Cullen, S. Sidney and S. C. Johnston, Inpatient statin use predicts improved ischemic stroke discharge disposition, Neurology, 2012, 78, 1678–1683.
M. T. Heafield, S. Fearn, G. B. Steventon, R. H. Waring, A. C. Williams and S. G. Sturman, Plasma cysteine and sulfate levels in patients with motor-neuron, parkinsons and alzheimers-disease, Neurosci. Lett., 1990, 110, 216–220.
L. El-Khairy, S. E. Vollset, H. Refsum and P. M. Ueland, Plasma total cysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine Study, Am. J. Clin. Nutr., 2003, 77, 467–472.
H. Bradley, A. Gough, R. S. Sokhi, A. Hassell, R. Waring and P. Emery, Sulfate metabolism is abnormal in patients with rheumatoid-arthritis - confirmation by in-vivo biochemical findings, J. Rheumatol., 1994, 21, 1192–1196.
X. Chen, Y. Zhou, X. J. Peng and J. Yoon, Fluorescent and colorimetric probes for detection of thiols, Chem. Soc. Rev., 2010, 39, 2120–2135.
Y. Ogasawara, Y. Mukai, T. Togawa, T. Suzuki, S. Tanabe and K. Ishii, Determination of plasma thiol bound to albumin using affinity chromatography and high-performance liquid chromatography with fluorescence detection: Ratio of cysteinyl albumin as a possible biomarker of oxidative stress, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2007, 845, 157–163.
P. R. Lima, W. J. R. Santos, R. D. S. Luz, F. S. Damos, A. B. Oliveira, M. O. F. Goulart and L. T. Kubota, An amperometric sensor based on electrochemically triggered reaction: Redox-active Ar-NO/Ar-NHOH from 4-nitrophthalonitrile-modified electrode for the low voltage cysteine detection, J. Electroanal. Chem., 2008, 612, 87–96.
G. Chen, L. Y. Zhang and J. Wang, Miniaturized capillary electrophoresis system with a carbon nanotube microelectrode for rapid separation and detection of thiols, Talanta, 2004, 64, 1018–1023.
W. H. Wang, O. Rusin, X. Y. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, Detection of homocysteine and cysteine, J. Am. Chem. Soc., 2005, 127, 15949–15958.
H. Matsuura, Y. Sato, O. Niwa and F. Mizutani, Electrochemical enzyme immunoassay of a peptide hormone at picomolar levels, Anal. Chem., 2005, 77, 4235–4240.
M. Rafii, R. Elango, G. Courtney-Martin, J. D. House, L. Fisher and P. B. Pencharz, High-throughput and simultaneous measurement of homocysteine and cysteine in human plasma and urine by liquid chromatography-electro spray tandem mass spectrometry, Anal. Biochem., 2007, 371, 71–81.
H. L. Li, J. L. Fan, J. Y. Wang, M. Z. Tian, J. J. Du, S. G. Sun, P. P. Sun and X. J. Peng, A fluorescent chemodosimeter specific for cysteine: effective discrimination of cysteine from homocysteine, Chem. Commun., 2009, 5904–5906.
P. Wang, J. Liu, X. Lv, Y. L. Liu, Y. Zhao and W. Guo, A Naphthalimide-Based Glyoxal Hydrazone for Selective Fluorescence Turn-On Sensing of Cys and Hcy, Org. Lett., 2012, 14, 520–523.
L. Y. Niu, Y. S. Guan, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, BODIPY-Based Ratiometric Fluorescent Sensor for Highly Selective Detection of Glutathione over Cysteine and Homocysteine, J. Am. Chem. Soc., 2012, 134, 18928–18931.
X. F. Yang, Y. X. Guo and R. M. Strongin, Conjugate Addition/Cyclization Sequence Enables Selective and Simultaneous Fluorescence Detection of Cysteine and Homocysteine, Angew. Chem., Int. Ed., 2011, 50, 10690–10693.
D. P. Li, J. F. Zhang, J. Cui, X. F. Ma, J. T. Liu, J. Y. Miao and B. X. Zhao, A ratiometric fluorescent probe for fast detection of hydrogen sulfide and recognition of biological thiols, Sens. Actuators, B, 2016, 234, 231–238.
L. Yi, H. Y. Li, L. Sun, L. L. Liu, C. H. Zhang and Z. Xi, A Highly Sensitive Fluorescence Probe for Fast Thiol-Quantification Assay of Glutathione Reductase, Angew. Chem., Int. Ed., 2009, 48, 4034–4037.
B. K. McMahon and T. Gunnlaugsson, Selective Detection of the Reduced Form of Glutathione (GSH) over the Oxidized (GSSG) Form Using a Combination of Glutathione Reductase and a Tb(III)-Cyclen Maleimide Based Lanthanide Luminescent ‘Switch On’ Assay, J. Am. Chem. Soc., 2012, 134, 10725–10728.
P. B. Viviana, C. A. Huerta-Aguilar, N. Singh and T. Pandiyan, Selective recognition of Cr3+ in multivitamin formulations in aqueous medium by fluorescent organicinorganic nanohybrids, Res. Chem. Intermed., 2018, 44, 3179–3197.
C. A. Huerta-Aguilar, T. Pandiyan, P. Raj, N. Singh and R. Zanella, Fluorescent organic nanoparticles (FONs) for the selective recognition of Zn2+: Applications to multivitamin formulations in aqueous medium, Sens. Actuators, B, 2016, 223, 59–67.
C. A. Huerta-Aguilar, P. Raj, P. Thangarasu and N. Singh, Fluorescent organic nanoparticles (FONs) for selective recognition of Al3+: application to bio-imaging for bacterial sample, RSC Adv., 2016, 6, 37944–37952.
C. A. Huerta-Aguilar, T. Pandiyan, N. Singh and N. Jayanthi, Three novel input logic gates supported by fluorescence studies: Organic nanoparticles (ONPs) as chemo-sensor for detection of Zn2+ and Al3+ in aqueous medium, Spectrochim. Acta, Part A, 2015, 146, 142–150.
C. A. H. Aguilar, A. B. P. Jimenez, A. R. Silva, N. Kaur, P. Thangarasu, J. M. V. Ramos and N. Singh, Organic-Inorganic Hybrid Nanoparticles for Bacterial Inhibition: Synthesis and Characterization of Doped and Undoped ONPs with Ag/Au NPs, Molecules, 2015, 20, 6002–6021.
A. Ravindran, M. Elavarasi, T. C. Prathna, A. M. Raichur, N. Chandrasekaran and A. Mukherjee, Selective colorimetric detection of nanomolar Cr(VI) in aqueous solutions using unmodified silver nanoparticles, Sens. Actuators, B, 2012, 166, 365–371.
Y. P. Zhang, J. Chen, L. Y. Bai, X. M. Zhou and L. M. Wang, Gold Nanoparticle-based Optical Probe for Quick Colorimetric Visualization of Cysteine, J. Chin. Chem. Soc., 2010, 57, 972–975.
A. Sugunan, C. Thanachayanont, J. Dutta and J. G. Hilborn, Heavy-metal ion sensors using chitosan-capped gold nanoparticles, Sci. Technol. Adv. Mater., 2005, 6, 335–340.
E. Ide, S. Angata, A. Hirose and K. F. Kobayashi, Metal–metal bonding process using Ag metallo-organic nanoparticles, Acta Mater., 2005, 53, 2385–2393.
C.-C. Shih, Y.-C. Chiu, W.-Y. Lee, J.-Y. Chen and W.-C. Chen, Conjugated Polymer Nanoparticles as Nano Floating Gate Electrets for High Performance Nonvolatile Organic Transistor Memory Devices, 2015, vol. 25, pp. 1511–1519.
S. Devi, B. Singh, A. K. Paul and S. Tyagi, Highly sensitive and selective detection of trinitrotoluene using cysteinecapped gold nanoparticles, Anal. Methods, 2016, 8, 4398–4405.
S. Xu, Y. Wang, Y. Sun, G. Shan, Y. Chen and Y. Liu, The detection of copper ions based on photothermal effect of cysteine modified Au nanorods, Sens. Actuators, B, 2017, 248, 761–768.
S. Verma, S. S. Amritphale and S. Das, Surfaces, Multifunctional application of cytosine for the synthesis of hybrid homogenized nano-sized rare earth oxide (Re2O3) and rare earth oxycarbonate (Re2O2CO3) (Re=Nd, Sm) Adv. Material Microwave Irradiation, 2017, vol. 53, pp. 444–451.
S. Verma, S. S. Amritphale and S. Das, Synchronising effect of microwave and cytosine for the synthesis of hybrid homogenised nanosized cerium oxide and cerium oxycarbonate hydrate material, J. Chem. Res., 2016, 40, 321–325.
Y. Zhang and Q. Zhong, Probing the binding between norbixin and dairy proteins by spectroscopy methods, Food Chem., 2013, 139, 611–616.
S. Jiang, H.-Z. Liu, W.-L. Cai, A.-m. Bai, Y. Ouyang and Y.-J. Hu, Quasi-spherical silver nanoparticles with high dispersity and uniform sizes: preparation, characterization and bioactivity in their interaction with bovine serum albumin, Luminescence, 2016, 31, 1146–1151.
A. Singh, A. Singh and N. Singh, A Cu(II) complex of an imidazolium-based ionic liquid: synthesis, X-ray structure and application in the selective electrochemical sensing of guanine, Dalton Trans., 2014, 43, 16283–16288.
V. D. Sotnikov, V. A. Zherdev and B. B. Dzantiev, Development and Application of a Label-Free Fluorescence Method for Determining the Composition of Gold Nanoparticle–Protein Conjugates, Int. J. Mol. Sci., 2015, 16, 907–923.
M. Ahumada, E. Lissi, A. M. Montagut, F. Valenzuela-Henriquez, N. L. Pacioni and E. I. Alarcon, Association models for binding of molecules to nanostructures, Analyst, 2017, 142, 2067–2089.
G. A. Crosby and J. N. Demas, Measurement of photoluminescence quantum yields. Review, J. Phys. Chem., 1971, 75, 991–1024.
K. Rurack and M. Spieles, Fluorescence Quantum Yields of a Series of Red and Near-Infrared Dyes Emitting at 600–1000 nm, Anal. Chem., 2011, 83, 1232–1242.
A. Balamurugan and H.-i. Lee, Aldoxime-Derived Water-Soluble Polymer for the Multiple Analyte Sensing: Consecutive and Selective Detection of Hg2+, Ag+, ClO–, and Cysteine in Aqueous Media, Macromolecules, 2015, 48, 3934–3940.
X. Liu, D. Yang, W. Chen, L. Yang, F. Qi and X. Song, A redemitting fluorescent probe for specific detection of cysteine over homocysteine and glutathione with a large Stokes shift, Sens. Actuators, B, 2016, 234, 27–33.
R. Na, M. Zhu, S. Fan, Z. Wang, X. Wu, J. Tang, J. Liu, Y. Wang and R. Hua, A Simple and Effective Ratiometric Fluorescent Probe for the Selective Detection of Cysteine and Homocysteine in Aqueous Media, Molecules, 2016, 21, 1023.
R. M. Jiang, H. Liu, M. Y. Liu, J. W. Tian, Q. Huang, H. Y. Huang, Y. Q. Wen, Q. Y. Cao, X. Y. Zhang and Y. Wei, A facile one-pot Mannich reaction for the construction of fluorescent polymeric nanoparticles with aggregationinduced emission feature and their biological imaging, Mater. Sci. Eng., C, 2017, 81, 416–421.
X. Y. Zhang, K. Wang, M. Y. Liu, X. Q. Zhang, L. Tao, Y. W. Chen and Y. Wei, Polymeric AIE-based nanoprobes for biomedical applications: recent advances and perspectives, Nanoscale, 2015, 7, 11486–11508.
L. C. Mao, M. Y. Liu, R. M. Jiang, Q. Huang, Y. F. Dai, J. W. Tian, Y. G. Shi, Y. Q. Wen, X. Y. Zhang and Y. Wei, The one-step acetalization reaction for construction of hyperbranched and biodegradable luminescent polymeric nanoparticles with aggregation-induced emission feature, Mater. Sci. Eng., C, 2017, 80, 543–548.
S. X. Yu, D. Z. Xu, Q. Wan, M. Y. Liu, J. W. Tian, Q. Huang, F. J. Deng, Y. Q. Wen, X. Y. Zhang and Y. Wei, Construction of biodegradable and biocompatible AIE-active fluorescent polymeric nanoparticles by Ce(IV)/HNO3 redox polymerization in aqueous solution, Mater. Sci. Eng., C, 2017, 78, 191–197.
J. W. Tian, R. M. Jiang, P. Gao, D. Z. Xu, L. C. Mao, G. J. Zeng, M. Y. Liu, F. J. Deng, X. Y. Zhang and Y. Wei, Synthesis and cell imaging applications of amphiphilic AIE-active poly(amino acid)s, Mater. Sci. Eng., C, 2017, 79, 563–569.
Q. Wan, R. M. Jiang, L. L. Guo, S. X. Yu, M. Y. Liu, J. W. Tian, G. Q. Liu, F. J. Deng, X. Y. Zhang and Y. Wei, Novel Strategy toward AIE-Active Fluorescent Polymeric Nanoparticles from Polysaccharides: Preparation and Cell Imaging, ACS Sustainable Chem. Eng., 2017, 5, 9955–9964.
Q. Wan, M. Y. Liu, L. C. Mao, R. M. Jiang, D. Z. Xu, H. Y. Huang, Y. F. Dai, F. J. Deng, X. Y. Zhang and Y. Wei, Preparation of PEGylated polymeric nanoprobes with aggregation-induced emission feature through the combination of chain transfer free radical polymerization and multicomponent reaction: Self-assembly, characterization and biological imaging applications, Mater. Sci. Eng., C, 2017, 72, 352–358.
X. Y. Zhang, S. Q. Wang, L. X. Xu, L. Feng, Y. Ji, L. Tao, S. X. Li and Y. Wei, Biocompatible polydopamine fluorescent organic nanoparticles: facile preparation and cell imaging, Nanoscale, 2012, 4, 5581–5584.
H. Y. Huang, D. Z. Xu, M. Y. Liu, R. M. Jiang, L. C. Mao, Q. Huang, Q. Wan, Y. Q. Wen, X. Y. Zhang and Y. Wei, Direct encapsulation of AIE-active dye with beta cyclodextrin terminated polymers: Self-assembly and biological imaging, Mater. Sci. Eng., C, 2017, 78, 862–867.
Z. Long, M. Y. Liu, L. C. Mao, G. J. Zeng, Q. Huang, H. Y. Huang, F. J. Deng, Y. Q. Wan, X. Y. Zhang and Y. Wei, One-step synthesis, self-assembly and bioimaging applications of adenosine triphosphate containing amphiphilies with aggregation-induced emission feature, Mater. Sci. Eng., C, 2017, 73, 252–256.
N. Bel Haj Mohamed, N. Ben Brahim, R. Mrad, M. Haouari, R. Ben Chaâbane and M. Negrerie, Use of MPA-capped CdS quantum dots for sensitive detection and quantification of Co2+ ions in aqueous solution, Anal. Chim. Acta, 2018, 1028, 50–58.
M. R. Smith, M. G. Boenzli, V. Hindagolla, J. Ding, J. M. Miller, J. E. Hutchison, J. A. Greenwood, H. Abeliovich and A. T. Bakalinsky, Identification of gold nanoparticleresistant mutants of Saccharomyces cerevisiae suggests a role for respiratory metabolism in mediating toxicity, Appl. Environ. Microbiol., 2013, 79, 728–733.
Z. V. Feng, I. L. Gunsolus, T. A. Qiu, K. R. Hurley, L. H. Nyberg, H. Frew, K. P. Johnson, A. M. Vartanian, L. M. Jacob, S. E. Lohse, M. D. Torelli, R. J. Hamers, C. J. Murphy and C. L. Haynes, Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria, Chem. Sci., 2015, 6, 5186–5196.
S. Chopra, J. Singh, N. Singh and N. Kaur, Fluorescent organic nanoparticles of tripodal receptor as sensors for HSO4- in aqueous medium: application to real sample analysis, Anal. Methods, 2014, 9030–9036.
W. Yang and W. J. Mortier, The use of global and local molecular-parameters for the analysis of the gas-phase basicity of amines, J. Am. Chem. Soc., 1986, 108, 5708–5711.
N.W. Alcock, P. Moore and H. A. A. Omar, Synthesis of a pyridine-containing tetra-aza macrocycle, 7-methyl-3,7,11,17-tetraazabicyclo 11.3.1 heptadeca-1(17),13,15-triene (L-1), and characterization of its nickel(II), copper(II), and zinc(II) complexes - reduction of the pyridine ring of Ni(L-1)2+ to give Ni (L-2)2+ (L-2=7-methyl-3,7,11,17-tetra-azabicyclo 11.3.1 heptadecane), and characterization of Ni(L-2)2+ by X-ray crystallographyp, J. Chem. Soc., Dalton Trans., 1986, 985–989.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9pp00060g
Rights and permissions
About this article
Cite this article
Huerta-Aguilar, C.A., Ramírez-Guzmán, B., Thangarasu, P. et al. Simultaneous recognition of cysteine and cytosine using thiophene-based organic nanoparticles decorated with Au NPs and bio-imaging of cells. Photochem Photobiol Sci 18, 1761–1772 (2019). https://doi.org/10.1039/c9pp00060g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00060g