Abstract
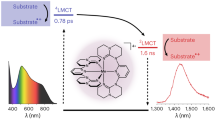
The early photophysical events occurring in the dinuclear metal complex [(ttb-terpy)(I)Ru(μ-dntpz)Ru (bpy)2]3+ (2; ttb-terpy = 4,4′,4″-tri-tert-butyl-terpy; bpy = 2,2′-bipyridine; dntpz = 2,5-di-(1,8-dinaphthyrid-2-yl)pyrazine) – a species containing the chromophoric {(bpy)2Ru(μ-dntpz)}2+ subunit and the catalytic {(I)(ttb-terpy)Ru(μ-dntpz)}+ unit, already reported to be able to perform photocatalytic water oxidation – have been studied by ultrafast pump–probe spectroscopy in acetonitrile solution. The model species [Ru(bpy)2(dntpz)]2+ (1), [(bpy)2Ru(μ-dntpz)Ru(bpy)2]4+ (3), and [(ttb-terpy)(I)Ru((μ-dntpz)Ru[(ttb-terpy)(I)]2+ (4) have also been studied. For completeness, the absorption spectra, redox behavior of 1–4 and the spectroelectrochemistry of the dinuclear species 2–4 have been investigated. The usual 3MLCT (metal-to-ligand charge transfer) decay, characterized by relatively long lifetimes on the ns timescale, takes place in 1 and 3, whose lowest-energy level involves a {(bpy)2Ru(dntpz)}2+ unit, whereas for 2 and 4, whose lowest-energy excited state involves a 3MLCT centered on the {(I)(ttb-terpy)Ru(μ-dntpz)}+ subunit, the excited-state lifetimes are on the ps timescale, possibly involving population of a low-lying 3MC (metal-centered) level. Compound 2 also exhibits a fast process, with a time constant of 170 fs, which is attributed to intercomponent energy transfer from the MLCT state centered in the {(bpy)2Ru(μ-dntpz)}2+ unit to the MLCT state involving the {(I)(ttb-terpy)Ru(μ-dntpz)}+ unit. Both the intercomponent energy transfer and the MLCT-to-MC activation process take place from non-equilibrated MLCT states.
Similar content being viewed by others
Notes and references
The topic is too vaste to be exhaustively quoted. For some representative articles, see: (a) J. H. Alstrum-Acevedo, M. K. Brennaman and T. J. Meyer, Inorg. Chem., 2005, 44, 6802; (b) R. Zong and R. P. Thummel, J. Am. Chem. Soc., 2005, 127, 12802; (c) I. Romero, M. Rodriguez, C. Sens, J. Mola, M. R. Kollipara, L. Francis, E. Mas-Marza, L. Escriche and A. Llobet, Inorg. Chem., 2008, 47, 1824; (d) J. J. Concepcion, J. W. Jurss, J. L. Templeton and T. J. Meyer, J. Am. Chem. Soc., 2008, 130, 16462; (e) F. Liu, J. J. Concepcion, J. W. Jurss, T. Cardolaccia, J. L. Templeton and T. J. Meyer, Inorg. Chem., 2008, 47, 1727; (f) H. W. Tseng, R. Zong, J. T. Muckerman and R. Thummel, Inorg. Chem., 2008, 47, 11763; (g) J. J. Concepcion, J. W. Jurss, J. L. Templeton and T. J. Meyer, J. Am. Chem. Soc., 2008, 130, 16462; (h) A. Sartorel, M. Carraro, G. Scorrano, R. De Zorzi, S. Geremia, N. D. McDaniel, S. Bernhard and M. Bonchio, J. Am. Chem. Soc., 2008, 130, 5006; (i) H. Yamazaki, A. Shouji, M. Kajita and M. Yagi, Coord. Chem. Rev., 2010, 254 ,2483; (j) S. Roeser, M. Z. Ertem, C. Cady, R. Lomoth, J. Benet-Buchholz, L. Hammarstroem, B. Sarkar, W. Kaim, C. J. Cramer and A. Llobet, Inorg. Chem., 2012, 51, 320.
(a) S. W. Gersten, G. J. Samuels and T. J. Meyer, J. Am. Chem. Soc., 1982, 104, 4029; (b) J. A. Gilbert, D. S. Eggleston, W. R. Murphy, D. A. Geselowitz, S. W. Gersten, D. J. Hodgson and T. J. Meyer, J. Am. Chem. Soc., 1985, 107, 3855.
See, for example: (a) L. Duan, F. Bozoglian, S. Mandal, B. Stewart, T. Privalov, A. Llobet and L. Sun, Nat. Chem., 2012, 4, 418; (b) S. Maji, L. Vigara, F. Cottone, F. Bozoglian, J. Benet-Bichholz and A. Llobet, Angew. Chem., Int. Ed., 2012, 51, 5967; (c) D. Moonshiram, I. Alperovich, J. J. Concepcion, T. J. Meyer and Y. Pushkar, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3765; (d) L. Kohler, N. Kaveevivitchai, R. Zong and R. P. Thummel, Inorg. Chem., 2014, 53, 912; (e) J. D. Blackmore, R. H. Crabtree and G. W. Brudvig, Chem. Rev., 2015, 115, 12974; (f) L. Tong and R. P. Thummel, Chem. Sci., 2016, 7, 6591; (g) Y. Tsubonouchi, S. Lin, A. R. Parent, G. W. Brudvig and K. Sakai, Chem. Commun., 2016, 52, 8018; (h) T. J. Meyer, M. W. Sheridan and B. D. Sherman, Chem. Soc. Rev., 2017, 46, 6148; (i) G. W. Brudvig and S. Campagna, Chem. Soc. Rev., 2017, 46, 6085; (j) D. W. Shaffer, Y. Xie and J. J. Concepcion, Chem. Soc. Rev., 2017, 46, 6170.
(a) J. J. Concepcion, M.-K. Tsai, J. T. Muckerman and T. J. Meyer, J. Am. Chem. Soc., 2010, 132, 1545; (b) D. E. Polyansky, J. T. Muckerman, J. Rochford, R. Zong, R. P. Thummel and E. Fujita, J. Am. Chem. Soc., 2011, 133, 14665; (c) A. Lewandowska-Andralojc, D. E. Polyansky, R. Zong, R. P. Thummel and E. Fujita, Phys. Chem. Chem. Phys., 2013, 15, 14058; (d) R. Matheu, M. Z. Ertem, J. Benet-Buchholz, E. Coronado, V. S. Batista, X. Sala and A. Llobet, J. Am. Chem. Soc., 2015, 137, 10786.
(a) L. Duan, L. Wang, A. K. Inge, A. Fisher, X. Zou and L. Sun, Inorg. Chem., 2013, 52, 7844; (b) D. W. Shaffer, Y. Xie, D. J. Szalda and J. J. Concepcion, Inorg. Chem., 2016, 55, 12024; (c) T. Fan, L. Duan, P. Huang, H. Chen, Q. Daniel, M. S. G. Ahlqyuist and L. Sun, ACS Catal., 2017, 7, 2956; (d) Y. Xie, D. W. Shaffer and J. J. Concepcion, Inorg. Chem., 2018, 57, 10533, and refs. therein.
(a) C. J. Richmond, R. Matheu, A. Poater, L. Falivene, J. Benet-Buchholz, X. Sala, L. Caballo and A. Llobet, Chem. – Eur. J., 2014, 20, 17282; (b) D. L. Ashford, M. K. Gish, A. K. Vannucci, M. K. Brennaman, J. L. Templeton, J. M. Papanikolas and T. J. Meyer, Chem. Rev., 2015, 115, 13006, and refs. therein.
N. Kaveevivitchai, R. Chitta, R. Zong, M. El Ojaimi and R. P. Thummel, J. Am. Chem. Soc., 2012, 134, 10721.
(a) M. K. Brennaman, R. J. Dillon, L. Alibabaei, M. K. Gish, C. J. Dares, D. L. Ashford, R. L. House, G. J. Meyer, J. M. Papanikolas and T. J. Meyer, J. Am. Chem. Soc., 2016, 138, 13085; (b) B. D. Sherman, Y. Xie, M. V. Sheridan, D. Wang, D. W. Shaffer, T. J. Meyer and J. J. Concepcion, ACS Energy Lett., 2017, 2, 124; (c) B. Shan, B. D. Sherman, C. M. Klug, A. Nayak, S. L. Marquard, Q. Liu, R. M. Bullock and T. J. Meyer, J. Phys. Chem. Lett., 2017, 8, 4374; (d) B. Shan, A. Nayak, M. K. Brennaman, M. Liu, S. L. Marquard, M. S. Eberhart and T. J. Meyer, J. Am. Chem. Soc., 2018, 140, 6493; (e) L. Wu, M. Eberhart, A. Nayak, M. K. Brennaman, B. Shan and T. J. Meyer, J. Am. Chem. Soc., 2018, 140, 15062.
J. T. Hewitt, J. J. Concepcion and N. H. Damrauer, J. Am. Chem. Soc., 2013, 135, 12500.
M. Chrzanowska, A. Katafias, O. Impert, A. Kozakiewicz, A. Surdykowski, P. Brzozowska, A. Zachl, R. Puchta and R. van Eldik, Dalton Trans., 2017, 46, 10264, and refs therein.
D. Brown, S. Muranjan and R. P. Thummel, Eur. J. Inorg. Chem., 2003, 3547.
(a) J. M. McCusker, Acc. Chem. Res., 2003, 36, 876; (b) M. Chergui, Acc. Chem. Res., 2015, 49, 801 and refs. therein.
(a) S. Campagna, F. Puntoriero, F. Nastasi, G. Bergamini and V. Balzani, Top. Curr. Chem., 2007, 280, 117, and refs. therein.
Vibrational cooling in the same time range have been reported for several transition metal polypyridine complexes, see for example: (a) A. El Nahhas, C. Consanti, A. M. Blanco-Rodriguez, K. M. Lancaster, O. Braem, A. Cannizzo, M. Towrie, I. Clarck, S. Zalis, M. Chergui and A. Vlcek, Inorg. Chem., 2011, 50, 2932; (b) F. Nastasi, F. Puntoriero, M. Natali, M. Mba, M. Maggini, P. Mussini, M. Panigati and S. Campagna, Photochem. Photobiol. Sci., 2015, 14, 909.
F. Puntoriero, F. Nastasi, M. Galletta and S. Campagna, in Comprehensive Inorganic Chemistry II, ed. J. Reedijk and K. Poeppelmeier, Elsevier, Oxford, 2013, vol. 8, pp. 255–337.
(a) T. J. Meyer, Pure Appl. Chem., 1986, 58, 1193, and refs. therein. (b) A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85.
(a) H. Berglund Baudin, J. Davidsson, S. Serroni, A. Juris, V. Balzani, S. Campagna and L. Hammarström, J. Phys. Chem. A, 2002, 106, 4312; (b) J. Andersson, F. Puntoriero, S. Serroni, A. Yartsev, T. Pascher, T. Polivka, S. Campagna and V. Sundström, Chem. Phys. Lett., 2004, 386, 336; (c) J. Andersson, F. Puntoriero, S. Serroni, A. Yartsev, T. Pascher, T. Polivka, S. Campagna and V. Sundström, Faraday Discuss., 2004, 127 ,295; (d) J. Larsen, F. Puntoriero, T. Pascher, N. McClenaghan, S. Campagna, E. Åkesson and V. Sundström, ChemPhysChem, 2007, 8, 2643.
(a) G. Denti, S. Campagna, L. Sabatino, S. Serroni, M. Ciano and V. Balzani, Inorg. Chem., 1990, 29, 4750; (b) A. Arrigo, G. La Ganga, F. Nastasi, S. Serroni, A. Santoro, M.-P. Santoni, M. Galletta, S. Campagna and F. Puntoriero, C. R. Chim., 2017, 20, 209.
(a) G. Giuffrida and S. Campagna, Coord. Chem. Rev., 1994, 135–136, 517; (b) M. D. Ward, Chem. Soc. Rev., 1995, 24, 121 and refs. therein.
M. Natali, S. Campagna and F. Scandola, Chem. Soc. Rev., 2014, 43, 4005.
T. Koopmans, Physica, 1934, 1, 104.
E. Jakubikova, W. Chen, D. N. Dattelbaum, F. N. Rein, R. C. Rocha, R. L. Martin and E. R. Batista, Inorg. Chem., 2009, 48, 10720.
B. S. Brunschwig, C. Creutz and N. Sutin, Chem. Soc. Rev., 2002, 31, 168 and refs. therein.
D. M. D’Alessandro and F. R. Keane, Chem. Soc. Rev., 2006, 35i, 424.
(a) M. Burian, Z. Syrgiannis, G. La Ganga, F. Puntoriero, M. Natali, F. Scandola, S. Campagna, M. Prato, M. Bonchio, H. Amenitsch and A. Sartorel, Inorg. Chim. Acta, 2017, 454, 171; (b) A. Arrigo, F. Puntoriero, G. La Ganga, S. Campagna, M. Burian, S. Bernstorff and H. Amenitsch, Chem, 2017, 3, 494.
(a) J. E. Moser and M. Graetzel, Chem. Phys., 1993, 176, 493; (b) Y. Tachibana, J. E. Moser, M. Grätzel, D. R. Klug and J. R. Durrant, Chem. Phys. Lett., 1997, 272, 489; (c) N. S. McCool, J. R. Swierk, C. T. Nemes, C. A. Schmuttenmaer and T. E. Mallouk, J. Phys. Chem. Lett., 2016, 7, 2930; (d) J. R. Swierk, N. S. McCool, C. T. Nemes, T. E. Mallouk and C. A. Schmuttenmaer, J. Phys. Chem. C, 2016, 120, 5940; (e) C. S. Ponseca, P. Chabera, J. Uhlig, P. Persson and V. Sundstroem, Chem. Rev., 2017, 117, 10940.
Author information
Authors and Affiliations
Additional information
Electronic supplementary information (ESI) available: General procedures, equipments and methods, synthesis and characterization details. See DOI: 10.1039/c8pp00530c
Rights and permissions
About this article
Cite this article
Nastasi, F., Santoro, A., Serroni, S. et al. Early photophysical events of a ruthenium(II) molecular dyad capable of performing photochemical water oxidation and of its model compounds. Photochem Photobiol Sci 18, 2164–2173 (2019). https://doi.org/10.1039/c8pp00530c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00530c