Abstract
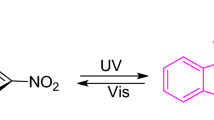
Carboxamidines functionalized with either a spiropyran or fulgimide photoswitch were prepared on multigram scales. The thermal, electrochemical, and photochemical ring isomerizations of these compounds were studied and the results compared with related systems. The photochemical isomerisations were found to be reversible and could be followed by 1H NMR and UV-vis spectroscopy. The spiropyran/merocyanine couple was thermally active and an activation enthalpy of 116 kJ mol−1 was measured for ring-opening. These measurements yielded an enthalpy difference of 25 kJ mol−1 between the open and closed states which is consistent with DFT calculations. DFT calculations predicted a charge transfer to the carboxamidine group upon ring closure in the fulgimide and a charge transfer from the carboxamidine group upon switching the spiropyran to the merocyanine form. This was confirmed experimentally by monitoring the change in the oxidation potential assigned to the carboxamidine group. The potential of these molecules to therefore act as a new class of photoresponsive ligands that can modulate the ligand field of a complex is discussed.
Similar content being viewed by others
References
R. Zheng, X. Mei, Z. Lin, Y. Zhao, W. Lv and Q. Ling, Strong CIE activity, multi-stimuli-responsive fluorescence and data storage application of new diphenyl maleimide derivatives, J. Mater. Chem. C, 2015, 3, 10242–10248.
L. Hu, Y. Duan, Z. Xu, J. Yuan, Y. Dong and T. Han, Stimuli-responsive fluorophores with aggregation-induced emission: implication for dual-channel optical data storage, J. Mater. Chem. C, 2016, 4, 5334–5341.
H. Gao, Y. Bi, J. Chen, L. Peng, K. Wen, P. Ji, W. Ren, X. Li, N. Zhang, J. Gao, Z. Chai and Y. Hu, Near-Infrared Light-Triggered Switchable Nanoparticles for Targeted Chemo/ Photothermal Cancer Therapy, ACS Appl. Mater. Interfaces, 2016, 8, 15103–15112.
X. An, A. Zhu, H. Luo, H. Ke, H. Chen and Y. Zhao, Rational Design of Multi-Stimuli-Responsive Nanoparticles for Precise Cancer Therapy, ACS Nano, 2016, 10, 5947–5958.
L. Zhang and L. Chen, Fluorescence Probe Based on Hybrid Mesoporous Silica/Quantum Dot/Molecularly Imprinted Polymer for Detection of Tetracycline, ACS Appl. Mater. Interfaces, 2016, 8, 16248–16256.
A. M. Lifschitz, R. M. Young, J. Mendez-Arroyo, C. M. McGuirk, M. R. Wasielewski and C. A. Mirkin, Cooperative Electronic and Structural Regulation in a Bioinspired Allosteric Photoredox Catalyst, Inorg. Chem., 2016, 55, 8301–8308.
S. Sun, X. Yu, Y. Guo, L. Chen, X. Wang and Z. Jiang, Temperature-Responsive Polyoxometalate Catalysts for DBT Desulfurization in One-Pot Oxidation Combined with Extraction, Catal. Surv. Asia, 2016, 20, 98–108.
Y. Nakabayashi and Y. Nosaka, OH Radical Formation at Distinct Faces of Rutile TiO2 Crystal in the Procedure of Photoelectrochemical Water Oxidation, J. Phys. Chem. C, 2013, 117, 23832–23839.
Á. Valdés, Z.-W. Qu, G.-J. Kroes, J. Rossmeisl and J. K. Norskov, Oxidation and Photo-Oxidation of Water on TiO2 Surface, J. Phys. Chem. C, 2008, 112, 9872–9879.
Z. Ding, G. Q. Lu and P. F. Greenfield, Role of the Crystallite Phase of TiO2 in Heterogeneous Photocatalysis for Phenol Oxidation in Water, J. Phys. Chem. B, 2000, 104, 4815–4820.
E. Krausz and J. Ferguson, in Progress in Inorganic Chemistry, ed. S.J. Lippard, John Wiley & Sons, Inc., 1989, pp. 293–390.
B. M. Neilson and C. W. Bielawski, Illuminating Photoswitchable Catalysis, ACS Catal., 2013, 3, 1874–1885.
M. Vlatkovic, B. S. L. Collins and B. L. Feringa, Dynamic Responsive Systems for Catalytic Function, Chem. - Eur. J., 2016, 22, 17080–17111.
D. Bléger and S. Hecht, Visible-Light-Activated Molecular Switches, Angew. Chem., Int. Ed., 2015, 54, 11338–11349.
B. M. Neilson, V. M. Lynch and C. W. Bielawski, Photoswitchable N-Heterocyclic Carbenes: Using Light to Modulate Electron-Donating Properties, Angew. Chem., Int. Ed., 2011, 50, 10322–10326.
B. M. Neilson and C. W. Bielawski, Photoswitchable Metal-Mediated Catalysis: Remotely Tuned Alkene and Alkyne Hydroborations, Organometallics, 2013, 32, 3121–3128.
B. M. Neilson and C. W. Bielawski, Photoswitchable Organocatalysis: Using Light To Modulate the Catalytic Activities of N-Heterocyclic Carbenes, J. Am. Chem. Soc., 2012, 134, 12693–12699.
M. M. Paquette, B. O. Patrick and N. L. Frank, Determining the Magnitude and Direction of Photoinduced Ligand Field Switching in Photochromic Metal-Organic Complexes: Molybdenum-Tetracarbonyl Spirooxazine Complexes, J. Am. Chem. Soc., 2011, 133, 10081–10093.
R. A. Kopelman, S. M. Snyder and N. L. Frank, Tunable Photochromism of Spirooxazines via Metal Coordination, J. Am. Chem. Soc., 2003, 125, 13684–13685.
R. A. Kopelman, M. M. Paquette and N. L. Frank, Photoprocesses and magnetic behavior of photochromic transition metal indoline[ phenanthrolinospirooxazine] complexes: Tunable photochromic materials, Inorg. Chim. Acta, 2008, 361, 3570–3576.
M. M. Paquette, R. A. Kopelman, E. Beitler and N. L. Frank, Incorporating optical bistability into a magnetically bistable system: a photochromic redox isomeric complex, Chem. Commun., 2009, 5424–5426.
M. Nihei, Y. Suzuki, N. Kimura, Y. Kera and H. Oshio, Bidirectional Photomagnetic Conversions in a SpinCrossover Complex with a Diarylethene Moiety, Chem. - Eur.J., 2013, 19, 6946–6949.
M. Milek, F. W. Heinemann and M. M. Khusniyarov, Spin Crossover Meets Diarylethenes: Efficient Photoswitching of Magnetic Properties in Solution at Room Temperature, Inorg. Chem., 2013, 52, 11585–11592.
C. Roux, J. Zarembowitch, B. Gallois, T. Granier and R. Claude, Toward Ligand-Driven Light-Induced Spin Changing. Influence of the Configuration of 4-Styrylpyridine (stpy) on the Magnetic Properties of FeII(stpy)4(NCS)2 Complexes. Crystal Structures of the SpinCrossover Species Fe(trans-stpy)4(NCS)2 and of the High- Spin Species Fe(cis-stpy)4(NCS)2, Inorg. Chem., 1994, 33, 2273–2279.
F. T. Edelmann, in Advances in Organometallic Chemistry, ed. A.F. Hill and M.J. Fink, Academic Press, 2008, vol. 57, pp. 183–352.
J. Barker and M. Kilner, The coordination chemistry of the amidine ligand, Coord. Chem. Rev., 1994, 133, 219–300.
Y. Yokoyama, Fulgides for Memories and Switches, Chem. Rev., 2000, 100, 1717–1740.
G. Berkovic, V. Krongauz and V. Weiss, Spiropyrans and Spirooxazines for Memories and Switches, Chem. Rev., 2000, 100, 1741–1754.
P. H. M. Budzelaar, A. B. van Oort and A. G. Orpen, ß-Diiminato Complexes of VIII and TiII* - Formation and Structure of Stable Paramagnetic Dialkylmetal Compounds, Eur.J. Inorg. Chem., 1998, 1998, 1485–1494.
Y. Liang, A. S. Dvornikov and P. M. Rentzepis, Photochemistry of photochromic 2-indolylfulgides with substituents at the 1'-position of the indolylmethylene moiety, J. Photochem. Photobiol., A, 2001, 146, 83–93.
E. I. Balmond, B. K. Tautges, A. L. Faulkner, V. W. Or, B. M. Hodur, J. T. Shaw and A. Y. Louie, Comparative Evaluation of Substituent Effect on the Photochromic Properties of Spiropyrans and Spirooxazines, J. Org. Chem., 2016, 81, 8744–8758.
C. J. Roxburgh, P. G. Sammes and A. Abdullah, Steric and electronically biasing substituent effects on the Photoreversibility of novel, 3'-, 5'- and 3-substituted indolospirobenzopyrans. Thermal evaluation using 1H NMR spectroscopy and Overhauser enhancement studies, Dyes Pigm., 2009, 83, 31–50.
H. Görner, Photochromism of nitrospiropyrans: effects of structure, solvent and temperature, Phys. Chem. Chem. Phys., 2001, 3, 416–423.
A. K. Chibisov and H. Görner, Photochromism of spirobenzopyranindolines and spironaphthopyranindolines, Phys. Chem. Chem. Phys., 2001, 3, 424–431.
Y. Sheng, J. Leszczynski, A. A. Garcia, R. Rosario, D. Gust and J. Springer, Comprehensive Theoretical Study of the Conversion Reactions of Spiropyrans: Substituent and Solvent Effects, J. Phys. Chem. B, 2004, 108, 16233–16243.
G. Tomasello, M. J. Bearpark, M. A. Robb, G. Orlandi and M. Garavelli, Significance of a Zwitterionic State for Fulgide Photochromism: Implications for the Design of Mimics, Angew. Chem., Int. Ed., 2010, 49, 2913–2916.
S. Uchida, S. Yamada, Y. Yokoyama and Y. Kurita, Steric Effects of Substituents on the Photochromism of Indolylfulgides, Bull. Chem. Soc. Jpn., 1995, 68, 1677–1682.
S. Uchida, Y. Yokoyama, J. Kiji, T. Okano and H. Kitamura, Electronic Effects of Substituents on Indole Nitrogen on the Photochromic Properties of Indolylfulgides, Bull. Chem. Soc. Jpn., 1995, 68, 2961–2967.
R. Chindam, H. M. Hoque, A. S. Ali, F. Z. Rafique and J. D. Gough, Theoretical assessment of indolylfulgimides and novel asymmetric di-indolylfulgimide photochromes, J. Photochem. Photobiol., A, 2014, 279, 38–46.
Y. Liang, A. S. Dvornikov and P. M. Rentzepis, Synthesis of novel photochromic fluorescing 2-indolylfulgimides, Tetrahedron Lett., 1999, 40, 8067–8069.
J. Andréasson, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, Molecular All-Photonic Encoder-Decoder, J. Am. Chem. Soc., 2008, 130, 11122–11128.
A. S. Dvornikov, Y. Liang, C. S. Cruse and P. M. Rentzepis, Spectroscopy and Kinetics of a Molecular Memory with Nondestructive Readout for Use in 2D and 3D Storage Systems, J. Phys. Chem. B, 2004, 108, 8652–8658.
L. Li, F.-Q. Bai, J. Wang and H.-X. Zhang, Theoretical investigation on the spectroscopy prosperities of four isomers of an encoder molecule FGDTE, Dyes Pigm., 2014, 107, 108–117.
Y. Liang, A. S. Dvornikov and P. M. Rentzepis, Synthesis of novel photochromic fluorescing 2-indolylfulgimides, Tetrahedron Lett., 1999, 40, 8067–8069.
G. Naren, S. Li and J. Andréasson, One-Time Password Generation and Two-Factor Authentication Using Molecules and Light, ChemPhysChem, 2017, 1729–1729.
P. Remón, M. Bälter, S. Li, J. Andréasson and U. Pischel, An All-Photonic Molecule-Based D Flip-Flop, J. Am. Chem. Soc., 2011, 133, 20742–20745.
A. Abdullah, T. G. Nevell, P. G. Sammes and C. J. Roxburgh, Unusual thermo(photo)chromic properties of some mononitro- and dinitro- substituted 3'-alkyl indolospirobenzopyrans, Dyes Pigm., 2015, 121, 57–72.
N. Sivasankaran and K. Palaninathan, Photochromic switchable pendant indolyl fulgimide polypyrrole, Polym. Degrad. Stab., 2013, 98, 1852–1861.
J. T. C. Wojtyk, A. Wasey, P. M. Kazmaier, S. Hoz and E. Buncel, Thermal Reversion Mechanism of N-Functionalized Merocyanines to Spiropyrans: A Solvatochromic, Solvatokinetic, and Semiempirical Study, J. Phys. Chem. A, 2000, 104, 9046–9055.
A. K. Chibisov and H. Görner, Complexes of spiropyranderived merocyanines with metal ions: relaxation kinetics, photochemistry and solvent effects, Chem. Phys., 1998, 237, 425–442.
M. Zanoni, S. Coleman, K. J. Fraser, R. Byrne, K. Wagner, S. Gambhir, D. L. Officer, G. G. Wallace and D. Diamond, Physicochemical study of spiropyran-terthiophene derivatives: photochemistry and thermodynamics, Phys. Chem. Chem. Phys., 2012, 14, 9112–9120.
Y. Shiraishi, K. Yamamoto, S. Sumiya and T. Hirai, Spiropyran as a reusable chemosensor for selective colorimetric detection of aromatic thiols, Phys. Chem. Chem. Phys., 2014, 16, 12137–12142.
B. Daoust and J. Lessard, Electrochemical behavior of amidine hydrochlorides and amidines, Can. J. Chem., 1995, 73, 362–374.
H. E. Abdou, A. A. Mohamed and J. P. Fackler, Synthesis, Characterization, Luminescence, and Electrochemistry of New Tetranuclear Gold(I) Amidinate Clusters: Au4[PhNC (Ph)NPh]4, Au4[PhNC(CH3)NPh]4, and Au4[ArNC(H)NAr]4, J. Cluster Sci., 2007, 18, 630–641.
M. A. Fox and J. R. Hurst, Electrochemically induced peri-cyclic reactions. A radical anionic cyclization, J. Am. Chem. Soc., 1984, 106, 7626–7627.
O. Ivashenko, J. T. van Herpt, P. Rudolf, B. L. Feringa and W. R. Browne, Oxidative electrochemical aryl C-C coupling of spiropyrans, Chem. Commun., 2013, 49, 6737–6739.
M. Campredon, G. Giusti, R. Guglielmetti, A. Samat, G. Gronchi, A. Alberti and M. Benaglia, Radical ions and germyloxyaminoxyls from nitrospiro[indoline-naphtho-pyrans]. A combined electrochemical and EPR study, J. Chem. Soc., Perkin Trans. 2, 1993, 2089–2094.
G. Armendáriz-Vidales, E. Martínez-González, D. Hernández-Melo, J. Tiburcio and C. Frontana, Electrochemical Characterization of Spiropyran Structures, Procedia Chem., 2014, 12, 41–46.
J. A. Mata, F. E. Hahn and E. Peris, Heterometallic complexes, tandem catalysis and catalytic cooperativity, Chem. Sci., 2014, 5, 1723–1732.
T. Hirao, Conjugated systems composed of transition metals and redox-active n-conjugated ligands, Coord. Chem. Rev., 2002, 226, 81–91.
A. M. Allgeier and C. A. Mirkin, Ligand Design for Electrochemically Controlling Stoichiometric and Catalytic Reactivity of Transition Metals, Angew. Chem., Int. Ed., 1998, 37, 894–908.
V. Lyaskovskyy and B. de Bruin, Redox Non-Innocent Ligands: Versatile New Tools to Control Catalytic Reactions, ACS Catal., 2012, 2, 270–279.
M. J. Preigh, M. T. Stauffer, F.-T. Lin and S. G. Weber, Anodic oxidation mechanism of a spiropyran, J. Chem. Soc., Faraday Trans., 1996, 92, 3991–3996.
Acknowledgements
We are grateful for financial support from the Robert A. Welch Foundation (D-1838, USA), Texas Tech University and from the National Science Foundation (NMR instrument grant CHE-1048553). The authors would like to thank Dr Daniel Unruh and the Texas Tech University X-ray Facility for the crystallographic information discussed herein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrews, M.C., Peng, P., Rajput, A. et al. Modulation of the carboxamidine redox potential through photoinduced spiropyran or fulgimide isomerisation. Photochem Photobiol Sci 17, 432–441 (2018). https://doi.org/10.1039/c7pp00347a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c7pp00347a