Abstract
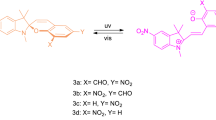
A novel hybrid compound whose molecule includes the photochromic spiropyran fragment and two fluorophores–coumarin and salicylideneimine–was synthesized by the reaction of two equivalents of 8-formyl-7-hydroxy-4-methylcoumarin with 5-amino-1,3,3-trimethyl-2-methyleneindoline. Its spectral-kinetic and fluorescence properties were studied in comparison with model compounds–7-hydroxy-4-methyl-coumarin, spiropyran of the coumarin type, and azomethinocoumarin in toluene and ethanol. It was shown that the hybrid compound exhibited photochromic transformations in both solvents. Different fluorescence emissions of the initial spirocycle and UV irradiation-induced merocyanine forms were observed. One green fluorescence emission with a maximum at 545 nm was observed for the initial closed form in toluene, and several emissions with maxima at 390 nm, 440 nm, 450 nm (violet), and 530 nm (green) appeared in ethanol. An additional red emission of the open merocyanine form in the region of 665 nm appeared after UV irradiation in both solvents. Reversible modulation of fluorescence in both the initial and photoinduced merocyanine forms in the process of photochromic transformations was discovered. It was shown that the fluorescence modulation of the hybrid compound in weakly polar toluene was caused by resonance energy transfer from the fluorescent fragment of the photochromic compound to the photoinduced merocyanine form, leading to the process of photochromic transformations. Reversible modulation of fluorescence in ethanol solutions was achieved through a photoinduced change in the ratio of the concentrations of conformers.
Similar content being viewed by others
References
K. Prakash, P. R. Sahoo, S. Kumar, A fast, highly selective and sensitive anion probe stemmed from anthracene-oxazine conjugation with CN− induced FRET, Dyes Pigm., 2017, 143, 393–400.
Q. Luo, F. Cao, C. Xiong, D.- Qu Dou, Hybrid cis/trans tetra-arylethenes with switchable aggregation-induced emission (aie) and reversible photochromism in the solution, PMMA film, solid powder, and single crystal, J. Org. Chem., 2017, 82, 10960–10967.
R. Wang, X. Dong, G. Liu, S. Pu, Substituent effects on the properties of photochromic hybrid diarylethenes with a naphthalene moiety, Spectrochim. Acta, Part A, 2015, 137, 1222–1230.
X. Yu, H. Liu, G. Liu, S. Pu, Efficient Synthesis and Properties of Hybrid Diarylethene Bearing a Pyrimidine Moiety, Adv. Mater. Res., 2014, 886, 159–163.
A. Bucko, S. Zielinska, E. Ortyl, M. Larkowska, R. Barille, Synthesis of organic–inorganic hybrid azobenzene materials for the preparation of nanofibers by electrospinning, Opt. Mater., 2014, 38, 179–187.
H. Liu, S. Pu, S. Cui, H. Li, Efficient Synthesis and Properties of Hybrid Diarylethene Bearing a Isoxazole Moiety, Adv. Mater. Res., 2012, 399–401, 1039–1042.
D. Jiang, G. Liu, W. Liu, Synthesis, photochromism properties of a hybrid diarylethene with hydroxyl group, Adv. Mater. Res., 2012, 393–395, 385–388.
F. Maurel, A. Perrier, D. Jacquemin, An ab initio simulation of a dithienylethene/phenoxynaphthacenequinone photochromic hybrid, J. Photochem. Photobiol., A, 2011, 218, 33–40.
A. Fihey, A. Perrier, W. R. Browne, D. Jacquemin, Multiphotochromic molecular systems, Chem. Soc. Rev., 2015, 44, 3719–3759.
K. Higashiguchi, K. Matsuda, N. Tanifuji, M. Irie, Fullcolor photochromism of a fused dithienylethene trimer, J. Am. Chem. Soc., 2005, 127, 8922–8923.
M. Tomasulo, E. Deniz, R. J. Alvarado, F. M. Raymo, Amplification of the Coloration Efficiency of Photochromic Oxazines, J. Phys. Chem. C, 2008, 112, 8038–8045.
G. Sevez, J. Gan, S. Delbaere, G. Vermeersch, L. Sanguinet, E. Levillain, J.-L. Pozzo, Photochromic performance of a dithienylethene-indolinooxazolidine hybrid, Photochem. Photobiol. Sci., 2010, 9, 131–135.
M. Frigoli, G. H. Mehl, Multiple adressing biphotochromic system, Angew. Chem., Int. Ed., 2005, 44, 5048–5052.
M. Tomasulo, S. L. Kaanumal, S. Sortino, F. M. Raymo, Synthesis and properties of benzophenone spiropyran and naphthalene spiropyran conjugates, J. Org. Chem., 2007, 72, 595–605.
A. N. Arkhipova, P. A. Panchenko, Yu. V. Fedorov, O. A. Fedorova, Relationship between the photochromic and fluorescent properties of 4-styryl derivatives of N-butyl-1,8-naphthalimide, Mendeleev Commun., 2017, 27, 53–55.
A. I. Shienok, N. A. Ivashina, L. S. Koltsova, N. L. Zaichenko, One-step synthesis of novel photochemically bifunctional compounds of the spiropyran class, Russ. Chem. Bull., Int. Ed., 2008, 57, 2437–2439.
P. P. Levin, A. S. Tatikolov, N. L. Zaichenko, A. I. Shienok, L. S. Koltsova, O. Yu. Oskina, I. R. Mardaleishvili, L. D. Popov, S. I. Levchenkov, A. A. Berlin, Kinetics of photochemical reactions of multifunctional hybrid compounds based on spironaphthoxazines upon photoexcitation with light of different wavelength, J. Photochem. Photobiol., A, 2013, 251, 141–147.
P. P. Levin, A. S. Tatikolov, N. L. Zaichenko, A. I. Shienok, L. S. Koltsova, I. R. Mardaleishvili, A. A. Berlin, Kinetics of photochemical reactions of biphotochromic compounds based on spironaphthopyran and enamine–conjugation effect, Photochem. Photobiol. Sci., 2016, 15, 382–388.
V. S. Marevtsev, N. L. Zaichenko, V. D. Ermakova, S. I. Beshenko, V. A. Linskii, A. T. Gradyushko, M. I. Cherkashin, Effect of electron-donating and electron-accepting substituents on photo- and thermochromic properties of indolinospiropyrans, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1980, 29, 1591–1596.
W. Sun, S. Li, R. Hu, Y. Qian, S. Wang, G. Yang, Understanding solvent effects on luminescent properties of a triple fluorescent ESIPT compound and application for white light emission, J. Phys. Chem. A, 2009, 113, 5888–5895.
M. I. Knyazhansky, A. V. Metelitsa, M. E. Kletskii, A. A. Millov, S. O. Besugliy, The structural transformations and photo-induced processes in salicylidene alkylimines, J. Mol. Struct., 2000, 526, 65–79.
M. Ziolek, J. Kubicki, A. Maciejewski, A. Naskrecki, Grabowska, Enol-reto tautomerism of aromatic photochromic Schiff base N,N′-bis(salicylisene)-p-phenylenediamine. Graund state equilibrium and excited state deactivation, J. Chem. Phys., 2006, 124, 124518–124528.
I. R. R. Mardaleishvili, L. S. Kol'tsova, N. L. Zaichenko, V. G. Sister, A. I. Shienok, P. P. Levin, A. S. Tatikolov, Spectral and luminescent properties of hydroxyazomethines of indoline spiropyrans, High Energy Chem., 2012, 46, 160–165.
D. J. Gale, J. F. K. Wilshire, Fibre-reactive basic dyes. I. Polymethine dyes, containing the N-chloroacetyl group, J. Soc. Dyers Color., 1974, 90, 97–100.
V. F. Traven, V. S. Miroshnikov, T. A. Chibisova, V. A. Barachevsky, O. V. Venidiktova, Yu. P. Strokach, Synthesis and structure of indoline spiropyrans of the coumarin series, Russ. Chem. Bull. Int. Ed., 2005, 54, 2417–2424.
T. Georgieva, N. Trendofilova, A. Aguino, H. Lischka, Excited state properties of 7-hydroxy-4-methylcoumarin in the gas phase and in solurions. A theoretical study, J. Phys. Chem. A, 2005, 109, 11860–11869.
J. Seixas de Melo, P. F. Fernandes, Spectroscopy and photophysics of 4- and 7-hydroxycoumarins and their thione analogs, J. Mol. Struct., 2001, 565–566, 69–78.
M. S. A. Abdel-Mottaleb, B. A. El-Sayed, M. M. Abo-Aly, M. Y. El-Kady, Fluorescence properties and excited state interactions of 7-hydroxy-4-methylcoumarin laser dye, J. Photochem. Photobiol., A, 1989, 46, 379–390.
M. Z. Zgierski, A. Grabowska, Photochromism of salycilideneaniline, J. Chem. Phys., 2000, 112, 6329–6337.
W. M. Fabian, L. Antonov, D. Nedeltcheva, F. S. Kamounah, P. J. Taylor, Tautomerism in Hydroxynaphthaldehyde Anils and Azo Analogues: a Combined Experimental and Computational Study, J. Phys. Chem. A, 2004, 108, 7603–7612.
M. Ziolek, M. Gil, J. A. Organero, A. Douhal, What is the difference between the dynamics of anion- and keto-type of photochromic salicylaldehyde azine, Phys. Chem. Chem. Phys., 2019, 12, 2107–2115.
N. Nizomov, A. U. Kholov, A. A. Ishchenko, V. V. Ishchenko, V. P. Khilia, Elecronic structure and spectral fluorescence properties of umbelliferone and herniarin, J. Appl. Spectrosc., 2007, 74, 626–634.
P. P. Levin, N. L. Zaichenko, A. I. Shienok, L. S. Koltsova, I. R. Mardaleishvili, A. S. Tatikolov, Spectral-kinetic characteristic of the photoisomerization products of naphthylmethylideneiminonaphthopyran induced by photolysis at different wavelengths, Russ. Chem. Bull. Int. Ed., 2012, 61, 532–538.
V. A. Barachevskii, R. E. Karpov, O. V. Venidiktova, T. M. Valova, Yu. P. Strokach, V. S. Miroshnikov, T. A. Chibisova, V. F. Traven, Photochromic properties of indoline spiropyrans of the coumarin series, Russ. Chem. Bull. Int. Ed., 2005, 54, 2425–2431.
V. Helms, in Principles of Computational Cell Biology, Wiley-VCH, Weinheim, 2008, p. 202.
Acknowledgments
This work was supported by the Federal Agency of Scientific Organizations (Agreement No 007-SO/Ch3363/26, topic 0082-2014-00154 AAAA-A17-117041210406-7) and by the RFBR (project no 16-03-00959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liubimov, A.V., Venidiktova, O.V., Valova, T.M. et al. Photochromic and luminescence properties of a hybrid compound based on indoline spiropyran of the coumarin type and azomethinocoumarin. Photochem Photobiol Sci 17, 1365–1375 (2018). https://doi.org/10.1039/c8pp00172c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c8pp00172c