Abstract
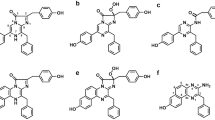
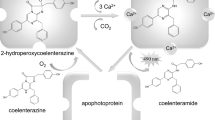
Bright bioluminescence of ctenophores is caused by Ca2+-regulated photoproteins. Although these photoproteins are functionally identical to and share many properties of cnidarian photoproteins, like aequorin and obelin, and retain the same spatial architecture, they are extremely sensitive to light, i.e. lose the ability to bioluminesce on exposure to light over the entire absorption spectrum. In addition, the degree of identity of their amino acid sequences with those of cnidarian photoproteins is only 29.4%. This suggests that the residues involved in bioluminescence of ctenophore and cnidarian photoproteins significantly differ. Here we describe the bioluminescent properties of berovin mutants with substitution of the residues located in the photoprotein internal cavity. Since the spatial structure of berovin bound with a substrate is not determined yet, to identify these residues we have modeled it with an accommodated substrate using the structures of some cnidarian Ca2+-regulated photoproteins with bound coelenterazine or coelenteramide as templates in order to obtain an adequate sampling and to take into account all possible conformers and variants for ligand–protein docking. Based on the impact of substitutions on the bioluminescent properties and model structures we speculate that within the internal cavity of ctenophore photoproteins, coelenterazine is bound as a 2-peroxy anion adduct which is stabilized owing to Coulomb interaction with a positively charged guanidinium group of Arg41 paired with Tyr204. In this case, the bioluminescence reaction is triggered by only calcium-induced conformational changes leading to the disturbance of charge–charge interaction.
Similar content being viewed by others
References
S. H. Haddock, M. A. Moline and J. F. Case, Bioluminescence in the sea, Annu. Rev. Mater. Sci., 2010, 2, 443–493.
E. A. Widder, Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity, Science, 2010, 328, 704–708.
S. V. Markova and E. S. Vysotski, Coelenterazine-dependent luciferases, Biochemistry, 2015, 80, 714–732.
O. Shimomura, in Bioluminescence: Chemical Principles and Methods, World Scientific Publishing Co., Singapore, 2006.
E. S. Vysotski, S. V. Markova and L. A. Frank, Calcium-regulated photoproteins of marine coelenterates, Mol. Biol., 2006, 40, 355–367.
O. Shimomura and F. H. Johnson, Regeneration of the photoprotein aequorin, Nature, 1975, 256, 236–238.
E. V. Eremeeva, P. V. Natashin, L. Song, Y. Zhou, W. J. van Berkel, Z. J. Liu and E. S. Vysotski, Oxygen activation of apo-obelin–coelenterazine complex, ChemBioChem, 2013, 14, 739–745.
O. Shimomura and F. H. Johnson, Structure of the light emitting moiety of aequorin, Biochemistry, 1972, 11, 1602–1608.
M. J. Cormier, K. Hori, Y. D. Karkhanis, J. M. Anderson, J. M. Wampler, J. G. Morin and J. W. Hastings, Evidence for similar biochemical requirements for bioluminescence among the coelenterates, J. Cell. Physiol., 1973, 81, 291–297.
E. S. Vysotski and J. Lee, Ca2+-regulated photoproteins: structural insight into the bioluminescence mechanism, Acc. Chem. Res., 2004, 37, 405–415.
J. F. Head, S. Inouye, K. Teranishi and O. Shimomura, The crystal structure of the photoprotein aequorin at 2.3 Å resolution, Nature, 2000, 405, 372–376.
Z. J. Liu, E. S. Vysotski, L. Deng, J. Lee, J. P. Rose and B. C. Wang, Atomic resolution structure of obelin: soaking with calcium enhances electron density of the second oxygen atom substituted at the C2-position of coelenterazine, Biochem. Biophys. Res. Commun., 2003, 311, 433–439.
M. S. Titushin, Y. Feng, G. A. Stepanyuk, Y. Li, S. V. Markova, S. Golz, B. C. Wang, J. Lee, J. Wang, E. S. Vysotski and Z. J. Liu, NMR-derived topology of a GFP-photoprotein energy transfer complex, J. Biol. Chem., 2010, 285, 40891–40900.
L. Burakova, P. Natashin, S. Markova, E. Eremeeva and E. Vysotsky, The C-terminal tyrosine deletion in mitrocomin increases its bioluminescent activity, Luminescence, 2014, 29, 84.
L. Deng, S. V. Markova, E. S. Vysotski, Z. J. Liu, J. Lee, J. Rose and B. C. Wang, Crystal structure of a Ca2+-discharged photoprotein: implications for mechanisms of the calcium trigger and bioluminescence, J. Biol. Chem., 2004, 279, 33647–33652.
Z. J. Liu, G. A. Stepanyuk, E. S. Vysotski, J. Lee, S. V. Markova, N. P. Malikova and B. C. Wang, Crystal structure of obelin after Ca2+-triggered bioluminescence suggests neutral coelenteramide as the primary excited state, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 2570–2575.
L. Deng, E. S. Vysotski, S. V. Markova, Z. J. Liu, J. Lee, J. Rose and B. C. Wang, All three Ca2+-binding loops of photoproteins bind calcium ions: the crystal structures of calcium-loaded apo-aequorin and apo-obelin, Protein Sci., 2005, 14, 663–675.
L. Deng, E. S. Vysotski, Z. J. Liu, S. V. Markova, N. P. Malikova, J. Lee, J. Rose and B. C. Wang, Structural basis for the emission of violet bioluminescence from a W92F obelin mutant, FEBS Lett., 2001, 506, 281–285.
E. S. Vysotski, Z. J. Liu, S. V. Markova, J. R. Blinks, L. Deng, L. A. Frank, M. Herko, N. P. Malikova, J. P. Rose, B. C. Wang and J. Lee, Violet bioluminescence and fast kinetics from W92F obelin: structure-based proposals for the bioluminescence triggering and the identification of the emitting species, Biochemistry, 2003, 42, 6013–6024.
P. V. Natashin, S. V. Markova, J. Lee, E. S. Vysotski and Z. J. Liu, Crystal structures of the F88Y obelin mutant before and after bioluminescence provide molecular insight into spectral tuning among hydromedusan photoproteins, FEBS J., 2014, 281, 1432–1445.
P. V. Natashin, W. Ding, E. V. Eremeeva, S. V. Markova, J. Lee, E. S. Vysotski and Z. J. Liu, Structures of the Ca2+-regulated photoprotein obelin Y138F mutant before and after bioluminescence support the catalytic function of a water molecule in the reaction, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2014, 70, 720–732.
E. S. Vysotski and J. Lee, Bioluminescent mechanism of Ca2+-regulated photoproteins from three-dimensional structures, in Luciferases and Fluorescent proteins: Principles and Advances in Biotechnology and Bioimaging, ed. V. R. Viviani and Y. Ohmiya, Transworld Research Network, Kerala, India, 2007, pp. 19–41.
M. S. Titushin, S. V. Markova, L. A. Frank, N. P. Malikova, G. A. Stepanyuk, J. Lee and E. S. Vysotski, Coelenterazine-binding protein of Renilla muelleri: cDNA cloning, overexpression, and characterization as a substrate of luciferase, Photochem. Photobiol. Sci., 2008, 7, 189–196.
G. A. Stepanyuk, Z. J. Liu, S. V. Markova, L. A. Frank, J. Lee, E. S. Vysotski and B. C. Wang, Crystal structure of coelenterazine-binding protein from Renilla muelleri at 1.7 Å: Why it is not a calcium-regulated photoprotein, Photochem. Photobiol. Sci., 2008, 7, 442–447.
G. A. Stepanyuk, Z. J. Liu, E. S. Vysotski, J. Lee, J. P. Rose and B. C. Wang, Structure based mechanism of the Ca2+-induced release of coelenterazine from the Renilla binding protein, Proteins, 2009, 74, 583–593.
G. A. Stepanyuk, Z. J. Liu, L. P. Burakova, J. Lee, J. Rose, E. S. Vysotski and B. C. Wang, Spatial structure of the novel light-sensitive photoprotein berovin from the ctenophore Beroe abyssicola in the Ca2+-loaded apoprotein conformation state, Biochim. Biophys. Acta, 2013, 1834, 2139–2146.
L. P. Burakova, P. V. Natashin, N. P. Malikova, F. Niu, M. Pu, E. S. Vysotski and Z. J. Liu, All Ca2+-binding loops of light-sensitive ctenophore photoprotein berovin bind magnesium ions: The spatial structure of Mg2+-loaded apo-berovin, J. Photochem. Photobiol., B, 2016, 154, 57–66.
S. V. Markova, L. P. Burakova, S. Golz, N. P. Malikova, L. A. Frank and E. S. Vysotski, The light-sensitive photoprotein berovin from the bioluminescent ctenophore Beroe abyssicola: a novel type of Ca2+-regulated photoprotein, FEBS J., 2012, 279, 856–870.
M. L. Powers, A. G. McDermott, N. C. Shaner and S. H. Haddock, Expression and characterization of the calcium-activated photoprotein from the ctenophore Bathocyroe fosteri: insights into light-sensitive photoproteins, Biochem. Biophys. Res. Commun., 2013, 431, 360–366.
G. A. Stepanyuk, S. Golz, S. V. Markova, L. A. Frank, J. Lee and E. S. Vysotski, Interchange of aequorin and obelin bioluminescence color is determined by substitution of one active site residue of each photoprotein, FEBS Lett., 2005, 579, 1008–1014.
E. V. Eremeeva, S. V. Markova, L. A. Frank, A. J. Visser, W. J. van Berkel and E. S. Vysotski, Bioluminescent and spectroscopic properties of His-Trp-Tyr triad mutants of obelin and aequorin, Photochem. Photobiol. Sci., 2013, 12, 1016–1024.
E. V. Eremeeva, S. V. Markova, W. J. van Berkel and E. S. Vysotski, Role of key residues of obelin in coelenterazine binding and conversion into 2–hydroperoxy adduct, J. Photochem. Photobiol., B, 2013, 127, 133–139.
E. V. Eremeeva, S. V. Markova, A. H. Westphal, A. J. W. G. Visser, W. J. H. van Berkel and E. S. Vysotski, The intrinsic fluorescence of apo-obelin and apo-aequorin and use of its quenching to characterize coelenterazine binding, FEBS Lett., 2009, 583, 1939–1944.
Y. J. M. Bollen, S. M. Nabuurs, W. J. H. van Berkel and C. P. M. van Mierlo, Last in, first out: the role of cofactor binding in flavodoxin folding, J. Biol. Chem., 2005, 280, 7836–7844.
H. Edelhoch, Spectroscopic determination of tryptophan and tyrosine in proteins, Biochemistry, 1967, 6, 1948–1954.
S. F. Sousa, P. A. Fernandes and M. J. Ramos, Protein-ligand docking: current status and future challenges, Proteins, 2006, 65, 15–26.
Z. J. Liu, E. S. Vysotski, C. J. Chen, J. P. Rose, J. Lee and B. C. Wang, Structure of the Ca2+-regulated photoprotein obelin at 1.7 Å resolution determined directly from its sulfur substructure, Protein Sci., 2000, 9, 2085–2093.
Y. Ohmiya, M. Ohashi and F. I. Tsuji, Two excited states in aequorin bioluminescence induced by tryptophan modification, FEBS Lett., 1992, 301, 197–201.
N. P. Malikova, G. A. Stepanyuk, L. A. Frank, S. V. Markova, E. S. Vysotski and J. Lee, Spectral tuning of obelin bioluminescence by mutations of Trp92, FEBS Lett., 2003, 554, 184–188.
L. A. Frank, V. V. Borisova, S. V. Markova, N. P. Malikova, G. A. Stepanyuk and E. S. Vysotski, Violet and greenish photoprotein obelin mutants for reporter applications in dual-color assay, Anal. Bioanal. Chem., 2008, 391, 2891–2896.
A. Mahdavi, R. H. Sajedi, S. Hosseinkhani, M. Taghdir and R. Sariri, Site-directed mutagenesis of photoprotein mnemiopsin: implication of some conserved residues in bioluminescence properties, Photochem. Photobiol. Sci., 2013, 12, 467–478.
M. R. Aghamaali, V. Jafarian, R. Sariri, M. Molakarimi, B. Rasti, M. Taghdir, R. H. Sajedi and S. Hosseinkhani, Cloning, sequencing, expression and structural investigation of mnemiopsin from Mnemiopsis leidyi: an attempt toward understanding Ca2+-regulated photoproteins, Protein J., 2011, 30, 566–574.
C. E. Schnitzler, K. Pang, M. L. Powers, A. M. Reitzel, J. F. Ryan, D. Simmons, T. Tada, M. Park, J. Gupta, S. Y. Brooks, R. W. Blakesley, S. Yokoyama, S. H. Haddock, M. Q. Martindale and A. D. Baxevanis, Genomic organization, evolution, and expression of photoprotein and opsin genes in Mnemiopsis leidyi: a new view of ctenophore photocytes, BMC Biol., 2012, 10, 107.
A. M. Loening, A. M. Wu and S. S. Gambhir, Red-shifted Renilla reniformis luciferase variants for imaging in living subjects, Nat. Methods, 2007, 4, 641–643.
G. A. Stepanyuk, J. Unch, N. P. Malikova, S. V. Markova, J. Lee and E. S. Vysotski, Coelenterazine-v ligated to Ca2+-triggered coelenterazine-binding protein is a stable and efficient substrate of the red-shifted mutant of Renilla muelleri luciferase, Anal. Bioanal. Chem., 2010, 398, 1809–1817.
K. Mori, S. Maki, H. Niwa, H. Ikeda and T. Hirano, Real light emitter in the bioluminescence of the calcium-activated photoproteins aequorin and obelin: light emission from the singlet-excited state of coelenteramide phenolate anion in a contact ion pair, Tetrahedron, 2006, 62, 6272–6288.
K. A. Schug and W. Lindner, Noncovalent binding between guanidinium and anionic groups: focus on biological- and synthetic-based arginine/guanidinium interactions with phosph[on]ate and sulf[on]ate residues, Chem. Rev., 2005, 105, 67–114.
T. K. Harris and G. J. Turner, Structural basis of perturbed pKa values of catalytic groups in enzyme active sites, UBMB Life, 2002, 53, 85–98.
T. Hirano, Y. Takahashi, H. Kondo, S. Maki, S. Kojima, H. Ikeda and H. Niwa, The reaction mechanism for the high quantum yield of Cypridina (Vargula) bioluminescence supported by the chemiluminescence of 6-aryl-2-methylimidazo[1,2-a]pyrazin-3(7H)-ones (Cypridina luciferin analogues), Photochem. Photobiol. Sci., 2008, 7, 197–207.
N. P. Malikova, L. P. Burakova, S. V. Markova and E. S. Vysotski, Characterization of hydromedusan Ca2+-regulated photoproteins as a tool for measurement of Ca2+ concentration, Anal. Bioanal. Chem., 2014, 406, 5715–5726.
J. W. Hastings, G. Mitchell, P. H. Mattingly, J. R. Blinks and M. Van Leeuwen, Response of aequorin bioluminescence to rapid changes in calcium concentration, Nature, 1969, 222, 1047–1050.
F. N. Tomilin, L. Yu. Antipina, E. S. Vysotski, S. G. Ovchinnikov and I. I. Gitelzon, Fluorescence of calcium-discharged obelin: The structure and molecular mechanism of emitter formation, Biochem. Biophys. Mol. Biol., 2008, 422, 279–284.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Fig. S1, Fig. S2, Fig. S3, and Table S1. See DOI: 10.1039/c6pp00050a
Rights and permissions
About this article
Cite this article
Burakova, L.P., Stepanyuk, G.A., Eremeeva, E.V. et al. Role of certain amino acid residues of the coelenterazine-binding cavity in bioluminescence of light-sensitive Ca2+-regulated photoprotein berovin. Photochem Photobiol Sci 15, 691–704 (2016). https://doi.org/10.1039/c6pp00050a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c6pp00050a