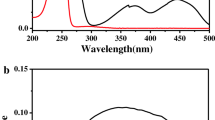
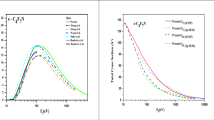
Abstract
Photochemical processes of 4-tert-butyl-4′-methoxydibenzoylmethane (Avobenzone, AB), 4-phenylbenzoylbenzoyl-, 4-phenylbenzoyl-2′-furanyl- and 4-phenylbenzoyl-2′-thenoylmethanes (PB@Ph, PB@F and PB@T, respectively) substituted with Br and Cl at the C2 position were studied by stationary and laser flash photolyses in solution. The absorption spectral features showed that the molecular structures of the halogenated diketones are in the keto forms while those of halogen-free diketones are in the enol forms. The excited singlet and triplet state energies were determined from the absorption and emission spectra. From the absorption spectral changes upon steady state photolysis of brominated diketones in ethanol, the corresponding halogen-free diketones were formed due to Br elimination being the major photochemical process. The determined quantum yields for the formation of the halogen-free diketones were independent of the amount of dissolved oxygen, indicating that the elimination process is an event in the excited singlet (S1) states. In contrast, from the observed absorption spectra obtained upon photolysis of chlorinated AB and PB@Ph, it was inferred that Norrish type I is the major photochemical reaction in the S1 states in acetonitrile. Chlorinated PB@F and PB@T were found to undergo Cl elimination in the S1 states in cyclohexane to form the corresponding halogen-free diketones. Laser photolysis studies of brominated AB in acetonitrile and ethanol provided a transient absorption spectrum ascribable to the Avobenzone radical (ABR) produced by debromination as the initial intermediate, followed by the AB formation in ethanol. The quenching rate constant of ABR by ethanol and the quantum yield of the AB formation via ABR were determined. These observations provided evidence that H-atom abstraction of ABR from ethanol is responsible for the AB formation. Conversely, laser flash photolysis of brominated and chlorinated PB@Ph, PB@F and PB@T demonstrated the formation of the triplet-triplet absorption spectra. No chemical reactions were found to occur in the triplet (T1) states. Two-color two-laser photolysis studies were carried out on the T1 state of chlorinated PB@Ph, PB@F and PB@T, resulting in the formation of the corresponding halogen-free diketones. These observations confirmed the occurrence of Cl elimination in the highly excited triplet (Tn, n ≥ 2) states. Based on the computed bond dissociation energies for the C-halogen and C-C bonds, switching mechanisms of dehalogenation and α-cleavage were discussed.
Similar content being viewed by others
Notes and References
P. Gacoin, Studies of the Triplet State of Carbonyl Compounds. I. Phosphorescence of β-Diketones, J. Chem. Phys., 1972, 57, 1418–1425.
A. J. Vila, C. M. Lagier and A. C. Olivieri, Proton transfer in solid 1-phenylbutane-1,3-dione and related 1,3-diones as studied by carbon-13 CPMAS NMR spectroscopy and AM1 calculations, J. Phys. Chem., 1991, 95, 5069–5073.
H. Gonzenbach, T. J. Hill and T. G. Truscott, The triplet energy levels of UVA and UVB sunscreens, J. Photochem. Photobiol., B, 1992, 16, 377–379.
N. M. Roscher, M. K. O. Lindemann, S. Bin Kong, C. G. Cho and P. Jiang, Photodecomposition of several compounds commonly used as sunscreen agents, J. Photochem. Photobiol., A, 1994, 80, 417–421.
W. Schwack and T. Rudolph, Photochemistry of dibenzoyl methane UVA filters Part 1, J. Photochem. Photobiol., B, 1995, 28, 229–234.
I. Andrae, A. Bringhen, F. Böhm, H. Gonzenbach, T. Hill, L. Mulroy and T. G. Truscott, A UVA filter (4-tert-butyl-4′-methoxydibenzoylmethane): photoprotection reflects photophysical properties, J. Photochem. Photobiol., B, 1997, 37, 147–150.
M. Dubois, P. Gilard, P. Tiercet, A. Deflandre and M. A. Lefebvre, Photoisomerisation of the sunscreen filter PARSOL©1789, J. Chim. Phys., 1998, 95, 388–394.
F. P. Gasparro, M. Mitchnick and J. F. Nash, A review of sunscreen safety and efficacy, Photochem. Photobiol., 1998, 68, 243–256.
A. Cantrell and D. J. McGarvey, Photochemical studies of 4-tert-butyl-4′-methoxydibenzoylmethane (BM-DBM), J. Photochem. Photobiol., B, 2001, 64, 117–122.
C. Eric and G. Bernard, Photostabilization of butyl methoxydibenzoylmethane (avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter, Photochem. Photobiol., 2001, 74, 401–406.
F. Wetz, C. Routaboul, D. Lavabre, J.-C. Garrigues, I. Rico-Lattes, I. Pernet and A. Denis, Photochemical behavior of a new long-chain UV absorber drived from 4-tert-butyl-4′-methoxydibenzoylmethane, Photochem. Photobiol., 2004, 80, 316–321.
E. Damiani, L. Rosati, R. Castagna, P. Carloni and L. Greci, Changes in ultraviolet absorbance and hence in protective efficacy against lipid peroxidation of organic sunscreens after UVA irradiation, J. Photochem. Photobiol., B, 2006, 82, 204–213.
D. Dondi, A. Albini and N. Serpone, Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection, Photochem. Photobiol. Sci., 2006, 5, 835–843.
A. Aspée, C. Aliaga and J. C. Scaiano, Transient enol isomers of dibenzoylmethane and avobenzone as efficient hydrogen donors toward a nitroxide pre-fluorescent probe, Photochem. Photobiol., 2007, 83, 481–485.
E. Damiani, W. Baschong and L. Greci, UV-Filter combinations under UV-A exposure: Concomitant quantification of over-all spectral stability and molecular integrity, J. Photochem. Photobiol., B, 2007, 87, 95–104.
S. P. Huong, E. Rocher, J.-D. Fourneron, L. Charles, V. Monnier, H. Bun and V. Andrieu, Photoreactivity of the sunscreen butylmethoxydibenzoylmethane (DBM) under various experimental conditions, J. Photochem. Photobiol., A, 2008, 196, 106–112.
G. J. Mturi and B. S. Martincigh, Photostability of the sunscreening agent 4-tert-butyl-4′-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity, J. Photochem. Photobiol., A, 2008, 200, 410–420.
C. Paris, V. Lhiaubet-Vallet, O. Jiménez, C. Trullas and M. Á. Miranda, A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter, Photochem. Photobiol., 2009, 85, 178–184.
M. Yamaji and M. Kida, Photothermal Tautomerization of a UV Sunscreen (4-tert-butyl-4′-methoxydibenzoylmethane) in acetonitrile studied by steady-state and laser flash photolysis, J. Phys. Chem. A, 2013, 117, 1946–1951.
N. Oguchi-Fujiyama, K. Miyazawa, A. Kikuchi and M. Yagi, Photophysical properties of dioctyl 4-methoxybenzylidenemalonate: UV-B absorber, Photochem. Photobiol. Sci., 2012, 11, 1528–1535.
M. Yamaji, C. Paris and M. Á. Miranda, Steady-state and laser flash photolysis studies on photochemical formation of 4-tert-butyl-4′-methoxydibenzoylmethane from its derivative via the Norrish Type II reaction in solution, J. Photochem. Photobiol., A, 2010, 209, 153–157.
M. Yamaji, S. Wakabayashi, S. Ueda, H. Shizuka and S. Tobita, Laser photolysis studies of endoergonic triplet energy transfer in solution by observing the carbon-sulfur bond cleavage of triplet-sensitized naphthylmethyl phenyl sulfide, Chem. Phys. Lett., 2003, 368, 41–48.
E. Cogné-Laage, J.-F. Allemand, O. Ruel, J.-B. Baudin, V. Croquette, M. Blanchard-Desce and L. Jullien, Diaroyl(methanato)boron difluoride compounds as medium-sensitive two-photon fluorescent probes, Chem.–Eur. J., 2004, 10, 1445–1455.
J. Košmrlj, M. Kočevar and S. Polanc, A new convenient bromination with KBrO3/KBr/Dowex®, Synth. Commun., 1996, 26, 3583–3592.
B. Košmrlj and B. Sket, Photocyclization of 2-chloro-substituted 1,3-diarylpropan-1,3-diones to flavones, Org. Lett., 2007, 9, 3993–3996.
E. W. Förster, K. H. Grellman and H. Linschitz, Reaction patterns and kinetics of the photoconversion of N-methyldiphenylamine to N-methycarbazole, J. Am. Chem. Soc., 1973, 95, 3108–3115.
M. Hoshino and M. Koizumi, Order of quencher participation in Photochemistry. I. Proton transfer from the excited p-hydroxybenzophenone in mixed solvents of cyclohexane and alcohols, Bull. Chem. Soc. Jpn., 1972, 45, 2731–2736.
M. Yamaji, Y. Aihara, T. Itoh, S. Tobita and H. Shizuka, Thermochemical profiles on hydrogen atom transfer from triplet naphthol and proton-induced electron transfer from triplet methoxynaphthalene to benzophenone via triplet exciplexes studied by laser flash photolysis, J. Phys. Chem., 1994, 98, 7014–7021.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, GAUSSIAN 09 (Revision C.01), Gaussian, Inc., Wallingford CT, 2010.
H. Okamoto, T. Takane, S. Gohda, Y. Kubozono, K. Sato, M. Yamaji and K. Satake, Efficient synthetic photocyclization for phenacenes using a continuous flow reactor, Chem. Lett., 2014, 43, 994–996.
S. L. Murov, I. Carmichael and G. L. Hug, Handbook of Photochemistry, Second Edition, Revised and Expanded, Marcel Dekker, Inc., New York, 2nd edn, Revised and expanded edn., 1993.
M. Yamaji, A. Kojima and S. Tobita, Stepwise laser photolysis studies of β-bond cleavage in highly excited triplet states of biphenyl derivatives having C-O bonds, J. Phys. Chem. A, 2007, 111, 770–776.
M. Yamaji, Stepwise two-color laser photolysis studies of α-cleavage in highly excited triplet states of α-acyl-4-phenylphenols, Photochem. Photobiol. Sci., 2008, 7, 711–717.
M. Yamaji, X. Cai, M. Sakamoto, M. Fujitsuka and T. Majima, Photodecomposition profiles of β-bond cleavage of phenylphenacyl derivatives in the higher triplet excited states during stepwise two-color two-laser flash Photolysis, J. Phys. Chem. A, 2008, 112, 11306–11311.
M. Yamaji, X. Cai, M. Sakamoto, M. Fujitsuka and T. Majima, α-Bond dissociation of p-phenylbenzoyl derivatives in the higher triplet excited state studied by two-color two-laser flash photolysis, J. Phys. Chem. A, 2009, 113, 1696–1703.
V. Vendrell-Criado, G. M. Rodríguez-Muñiz, M. Yamaji, V. Lhiaubet-Vallet, M. C. Cuquerella and M. A. Miranda, Two-photon chemistry from upper triplet states of thymine, J. Am. Chem. Soc., 2013, 135, 16714–16719.
W. G. Dauben, L. Salem and N. J. Turro, A classification of photochemical reactions, Acc. Chem. Res., 1975, 8, 41–54.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Electronic supplementary information (ESI) available: Absorption and emission spectra, absorption spectral changes upon steady photolysis, transient absorption spectra and results of DFT calculation for the heat of formation. See DOI: 10.1039/c5pp00211g
Rights and permissions
About this article
Cite this article
Yamaji, M., Suwa, Y., Shimokawa, R. et al. Photochemical reactions of halogenated aromatic 1,3-diketones in solution studied by steady state, one- and two-color laser flash photolyses. Photochem Photobiol Sci 14, 1673–1684 (2015). https://doi.org/10.1039/c5pp00211g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00211g