Abstract
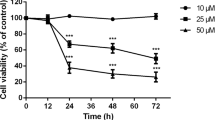
Purpose: Blue light has been previously demonstrated to induce injury of retinal cells. The cellular responses to nonlethal blue light exposure for each type of retinal cell are of particular interest but remain undetermined. Based on the doses of blue light reported in previous research to be nonlethal to retinal pigment epithelial cells, here we investigated whether and to what extent such doses of blue light are cytotoxic to staurosporine-differentiated RGC-5 cells. Methods: RGC-5 cells were differentiated for 24 hours using 200 nM staurosporine. The resulting cells were cultured and exposed to blue light at three different energy levels (1, 10, and 50 J cm-2). Cellular morphologies were investigated with an inverted microscope and cell viability was assessed with a Cell Counting Kit-8 (CCK-8) assay. The generation of intracellular reactive oxygen species (ROS) was evaluated by H2DCFDA. After loading of MitoTracker Green FM dye, the mitochondrial contents were analyzed using flow cytometry. The lactate dehydrogenase (LDH) activities in the media were also measured. The level of lipid peroxidation was determined by measuring the amount of malondialdehyde (MDA). Results: Treatment of the cells for 24 hours with 200 nM staurosporine successfully induced the differentiation of RGC-5 cells. No morphological changes were observed in the ssdRGC-5 cells exposed to blue light at 50 J cm-2, which was the highest energy level tested. Exposure of the ssdRGC-5 cells to this energy level of blue light did, however, decrease their numbers by approximately 72.1% compared to the numbers of such cells found after being left in the dark. Remarkably, the levels of ROS generation and mitochondrial contents were, respectively, increased to 142% and 118% of those of the control by a 10 J cm-2 exposure of blue light. The LDH activities and MDA levels exhibited no obvious changes in the blue light-exposed ssdRGC-5 cells compared to the control cells. Conclusions: In vitro nonlethal blue light exposure led to cellular damage of staurosporine-differentiated RGC-5 cells. These increases in oxidative stress and mitochondrial content were the early steps of the cellular response to the exposure of relatively low doses (10 J cm-2) of blue light.
Similar content being viewed by others
References
P. V. Algvere, J. Marshall and S. Seregard, Age-related maculopathy and the impact of blue light hazard, Acta Ophthalmol. Scand., 2006, 84, 4–15.
S. Ebert, Y. Walczak, C. Reme and T. Langmann, Microglial activation and transcriptomic changes in the blue light-exposed mouse retina, Adv. Exp. Med. Biol., 2012, 723, 619–632.
B. W. van der Burght, M. Hansen, J. Olsen, J. Zhou, Y. Wu, M. H. Nissen and J. R. Sparrow, Early changes in gene expression induced by blue light irradiation of A2E-laden retinal pigment epithelial cells, Acta Ophthalmol., 2013, 91, e537–e545.
D. Bennet, M. G. Kim and S. Kim, Light-induced anatomical alterations in retinal cells, Anal. Biochem., 2013, 436, 84–92.
M. L. Circu and T. Y. Aw, Reactive oxygen species, cellular redox systems, and apoptosis, Free Radicals Biol. Med., 2010, 48, 749–762.
D. Garcia-Ayuso, M. Salinas-Navarro, M. Agudo, N. Cuenca, I. Pinilla, M. Vidal-Sanz, M. P. Villegas-Perez, Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina, Exp. Eye Res., 2010, 91, 800–810.
D. Garcia-Ayuso, M. Salinas-Navarro, M. Agudo-Barriuso, L. Alarcon-Martinez, M. Vidal-Sanz, M. P. Villegas-Perez, Retinal ganglion cell axonal compression by retinal vessels in light-induced retinal degeneration, Mol. Vis., 2011, 17, 1716–1733.
C. Huang, P. Zhang, W. Wang, Y. Xu, M. Wang, X. Chen and X. Dong, Long-term blue light exposure induces RGC-5 cell death in vitro: involvement of mitochondria-dependent apoptosis, oxidative stress, and MAPK signaling pathways, Apoptosis, 2014, 19, 922–932.
M. A. Marco-Gomariz, N. Hurtado-Montalban, M. Vidal-Sanz, R. D. Lund, M. P. Villegas-Perez, Phototoxic-induced photoreceptor degeneration causes retinal ganglion cell degeneration in pigmented rats, J. Comp. Neurol., 2006, 498, 163–179.
S. del Olmo-Aguado, A. G. Manso and N. N. Osborne, Light might directly affect retinal ganglion cell mitochondria to potentially influence function, Photochem. Photobiol., 2012, 88, 1346–1355.
A. J. Moorhouse, S. Li, R. M. Vickery, M. A. Hill and J. W. Morley, A patch-clamp investigation of membrane currents in a novel mammalian retinal ganglion cell line, Brain Res., 2004, 1003, 205–208.
N. J. Van Bergen, J. P. Wood, G. Chidlow, I. A. Trounce, R. J. Casson, W. K. Ju, R. N. Weinreb and J. G. Crowston, Recharacterization of the RGC-5 retinal ganglion cell line, Invest Ophthalmol. Vis. Sci., 2009, 50, 4267–4272.
R. R. Krishnamoorthy, P. Agarwal, G. Prasanna, K. Vopat, W. Lambert, H. J. Sheedlo, I. H. Pang, D. Shade, R. J. Wordinger, T. Yorio, A. F. Clark and N. Agarwal, Characterization of a transformed rat retinal ganglion cell line, Brain Res. Mol. Brain Res., 2001, 86, 1–12.
C. J. Lieven, L. E. Millet, M. J. Hoegger and L. A. Levin, Induction of axon and dendrite formation during early RGC-5 cell differentiation, Exp. Eye Res., 2007, 85, 678–683.
L. J. Frassetto, C. R. Schlieve, C. J. Lieven, A. A. Utter, M. V. Jones, N. Agarwal and L. A. Levin, Kinase-dependent differentiation of a retinal ganglion cell precursor, Invest Ophthalmol. Vis. Sci., 2006, 47, 427–438.
B. R. Schwechter, L. E. Millet and L. A. Levin, Histone deacetylase inhibition-mediated differentiation of RGC-5 cells and interaction with survival, Invest Ophthalmol. Vis. Sci., 2007, 48, 2845–2857.
M. Schallenberg, P. Charalambous and S. Thanos, GM-CSF regulates the ERK1/2 pathways and protects injured retinal ganglion cells from induced death, Exp. Eye Res., 2009, 89, 665–677.
P. S. Ganapathy, Y. Dun, Y. Ha, J. Duplantier, J. B. Allen, A. Farooq, B. R. Bozard and S. B. Smith, Sensitivity of staurosporine-induced differentiated RGC-5 cells to homocysteine, Curr. Eye Res., 2010, 35, 80–90.
P. S. Nieto, V. A. Acosta-Rodriguez, D. J. Valdez and M. E. Guido, Differential responses of the mammalian retinal ganglion cell line RGC-5 to physiological stimuli and trophic factors, Neurochem. Int., 2010, 57, 216–226.
T. Nakanishi-Ueda, H. J. Majima, K. Watanabe, T. Ueda, H. P. Indo, S. Suenaga, T. Hisamitsu, T. Ozawa, H. Yasuhara and R. Koide, Blue LED light exposure develops intracellular reactive oxygen species, lipid peroxidation, and subsequent cellular injuries in cultured bovine retinal pigment epithelial cells, Free Radical Res., 2013, 47, 774–780.
T. Yu, J. L. Robotham and Y. Yoon, Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 2653–2658.
C. Roehlecke, A. Schaller, L. Knels and R. H. Funk, The influence of sublethal blue light exposure on human RPE cells, Mol. Vis., 2009, 15, 1929–1938.
A. Y. Andreyev, Y. E. Kushnareva and A. A. Starkov, Mitochondrial metabolism of reactive oxygen species, Biochemistry, 2005, 70, 200–214.
J. F. Turrens, Mitochondrial formation of reactive oxygen species, J. Physiol., 2003, 552, 335–344.
R. S. Balaban, S. Nemoto and T. Finkel, Mitochondria, oxidants, and aging, Cell, 2005, 120, 483–495.
V. Adam-Vizi and C. Chinopoulos, Bioenergetics and the formation of mitochondrial reactive oxygen species, Trends Pharmacol. Sci., 2006, 27, 639–645.
I. G. Onyango, J. Lu, M. Rodova, E. Lezi, A. B. Crafter and R. H. Swerdlow, Regulation of neuron mitochondrial biogenesis and relevance to brain health, Biochim. Biophys. Acta, 2010, 1802, 228–234.
S. Chen, Q. Fan, A. Li, D. Liao, J. Ge, A. M. Laties and X. Zhang, Dynamic mobilization of PGC-1alpha mediates mitochondrial biogenesis for the protection of RGC-5 cells by resveratrol during serum deprivation, Apoptosis, 2013, 18, 786–799.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, P., Huang, C., Wang, W. et al. Early changes in staurosporine-induced differentiated RGC-5 cells indicate cellular injury response to nonlethal blue light exposure. Photochem Photobiol Sci 14, 1093–1099 (2015). https://doi.org/10.1039/c4pp00456f
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c4pp00456f