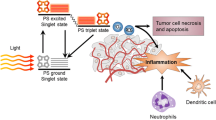
Abstract
Photodynamic therapy (PDT) has been used as a cancer therapy for forty years but has not advanced to a mainstream cancer treatment. Although it has been shown to be an efficient way to destroy local tumors by a combination of non-toxic dyes and harmless visible light, it is its additional effects in mediating the stimulation of the host immune system that gives PDT great potential to become more widely used. Although the stimulation of tumor-specific cytotoxic T-cells that can destroy distant tumor deposits after PDT has been reported in some animal models, it remains the exception rather than the rule. This realization has prompted several investigators to test various combination approaches that could potentiate the immune recognition of tumor antigens that have been released after PDT. This review will cover these combination approaches using immunostimulants including various microbial preparations that activate Toll-like receptors and other receptors for pathogen-associated molecular patterns, cytokines growth factors, and approaches that target regulatory T-cells. We believe that by understanding the methods employed by tumors to evade immune response and neutralizing them, more precise ways of potentiating PDT-induced immunity can be devised.
Similar content being viewed by others
References
M. R. Hamblin and P. Mroz, Advances in photodynamic therapy: basic, translational and clinical, Artech House, Norwood, MA, 2008.
A. F. Cruess, G. Zlateva, A. M. Pleil and B. Wirostko, Photodynamic therapy with verteporfin in age-related macular degeneration: a systematic review of efficacy, safety, treatment modifications and pharmacoeconomic properties, Acta Ophthalmol., 2009, 87, 118–132.
T. Dai, Y. Y. Huang and M. R. Hamblin, Photodynamic therapy for localized infections-State of the art, Photodiagn. Photodyn. Ther., 2009, 6, 170–188.
T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, Photodynamic therapy, J. Natl. Cancer Inst., 1998, 90, 889–905.
D. E. Dolmans, D. Fukumura and R. K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer, 2003, 3, 380–387.
M. Ochsner, Photophysical and photobiological processes in the photodynamic therapy of tumours, J. Photochem. Photobiol., B, 1997, 39, 1–18.
A. P. Castano, T. N. Demidova and M. R. Hamblin, Mechanisms in photodynamic therapy: part three -photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction, Photodiagn. Photodyn. Ther., 2005, 2, 91–106.
A. P. Castano, T. N. Demidova and M. R. Hamblin, Mechanisms in photodynamic therapy: part one–photosensitizers, photochemistry and cellular localization, Photodiagn. Photodyn. Ther., 2004, 1, 279–293.
M. F. Zuluaga and N. Lange, Combination of photodynamic therapy with anti-cancer agents, Curr. Med. Chem., 2008, 15, 1655–1673.
C. J. Gomer, S. W. Ryter, A. Ferrario, N. Rucker, S. Wong and A. M. Fisher, Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins, Cancer Res., 1996, 56, 2355–2360.
A. D. Garg, D. Nowis, J. Golab and P. Agostinis, Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity, Apoptosis, 2010, 15, 1050–1071.
M. Kwitniewski, A. Juzeniene, R. Glosnicka and J. Moan, Immunotherapy: a way to improve the therapeutic outcome of photodynamic therapy?, Photochem. Photobiol. Sci., 2008, 7, 1011–1017.
T. Mori, M. Okumura, M. Matsuura, K. Ueno, S. Tokura, Y. Okamoto, S. Minami and T. Fujinaga, Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro, Biomaterials, 1997, 18, 947–951.
H. O. Pae, W. G. Seo, N. Y. Kim, G. S. Oh, G. E. Kim, Y. H. Kim, H. J. Kwak, Y. G. Yun, C. D. Jun and H. T. Chung, Induction of granulocytic differentiation in acute promyelocytic leukemia cells (HL-60) by watersoluble chitosan oligomer, Leuk. Res., 2001, 25, 339–346.
M.-S. Kim, M.-J. Sung, S.-B. Seo, S.-J. Yoo, W.-K. Lim and H.-M. Kim, Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta. peptide and interleukin-1 beta, Neurosci. Lett., 2002, 321, 105–109.
W. R. Chen, M. Korbelik, K. E. Bartels, H. Liu, J. Sun and R. E. Nordquist, Enhancement of laser cancer treatment by a chitosanderived immunoadjuvant, Photochem. Photobiol., 2005, 81, 190–195.
J. M. Yuhas, N. H. Pazmino and E. Wagner, Development of concomitant immunity in mice bearing the weakly immunogenic line 1 lung carcinoma, Cancer Res., 1975, 35, 237–241.
W. T. Bradner, D. A. Clarke and C. C. Stock, Stimulation of host defense against experimental cancer. I. Zymosan and sarcoma 180 in mice, Cancer Res., 1958, 18, 347–351.
P. R. Taylor, G. D. Brown, D. M. Reid, J. A. Willment, L. Martinez-Pomares, S. Gordon and S. Y. Wong, The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages, J.Immunol., 2002, 169, 3876–3882.
Y. Y. Maeda and G. Chihara, The effects of neonatal thymectomy on the antitumour activity of lentinan, carboxymethylpachymaran and zymosan, and their effects on various immune responses, Int. J. Cancer, 1973, 11, 153–161.
A. Roeder, C. J. Kirschning, R. A. Rupec, M. Schaller, G. Weindl and H. C. Korting, Toll-like receptors as key mediators in innate antifungal immunity, Med. Mycol., 2004, 42, 485–498.
G. Krosl, M. Korbelik, J. Krosl and G. J. Dougherty, Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor, Cancer Res., 1996, 56, 3281–3286.
M. Korbelik, J. Sun, I. Cecic and K. Serrano, Adjuvant treatment for complement activation increases the effectiveness of photodynamic therapy of solid tumors, Photochem. Photobiol. Sci., 2004, 3, 812–816.
U. Winters, S. Daayana, J. T. Lear, A. E. Tomlinson, E. Elkord, P. L. Stern and H. C. Kitchener, Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia, Clin. Cancer Res., 2008, 14, 5292–5299.
W. T. Schaiff and P. R. Eisenberg, Direct induction of complement activation by pharmacologic activation of plasminogen, Coron. Artery Dis., 1997, 8, 9–18.
E. S. Abdel-Hady, P. Martin-Hirsch, M. Duggan-Keen, P. L. Stern, J. V. Moore, G. Corbitt, H. C. Kitchener and I. N. Hampson, Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy, Cancer Res., 2001, 61, 192–196.
P. Hillemanns, M. Untch, C. Dannecker, R. Baumgartner, H. Stepp, J. Diebold, H. Weingandt, F. Prove and M. Korell, Photodynamic therapy of vulvar intraepithelial neoplasia using 5-aminolevulinic acid, Int. J. Cancer, 2000, 85, 649–653.
P. L. Martin-Hirsch, C. Whitehurst, C. H. Buckley, J. V. Moore and H. C. Kitchener, Photodynamic treatment for lower genital tract intraepithelial neoplasia, Lancet, 1998, 351, 1403.
A. A. Gaspari, Mechanism of action and other potential roles of an immune response modifier, Quadrant HealthCom, Plainview, NY, 2007.
M. A. Stanley, Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential, Clin. Exp. Dermatol., 2002, 27, 571–577.
J. Moan and S. Sommer, Uptake of the components of hematoporphyrin derivative by cells and tumours, Cancer Lett., 1983, 21, 167–174.
A. P. Castano and M. R. Hamblin, Enhancing photodynamic therapy of a metastatic mouse breast cancer by immune stimulation, in W. R. Chen, (Ed.), Biophotonics and Immune Responses, The International Society for Optical Engineering, Bellingham, WA, San Jose, 2006, pp. 608–703.
M. Urano, M. Taradi and S. K. Taradi, Enhancement of the thermal response of murine tumour and normal tissues by a streptococcal preparation, OK-432 (Picibanil), Int. J. Hyperthermia, 1991, 7, 113–123.
S. S. Okamoto and H. R. Shimizu, Studies on the anticancer and streptolysin-S′ forming ability of hemolytic streptococci, Jpn. J. Microbiol., 1976, 11.
T. Sato, Y. Midorikawa, T. Yamashita, A. Araki and F. Sendo, The neutrophil as an information transmitter in tumor inhibition by a streptococcal biological response modifier, OK-432, Cancer Immunol. Immunother., 1996, 43, 77–86.
T. M. Katano M, New approach to management of malignant ascites with a streptococcal preparation, OK-432. II. Intraperitoneal inflammatory cell-mediated tumor cell destruction, Surgery, 1983, 93.
M. Uehara, K. Sano, Z.-L. Wang, J. Sekine, H. Ikeda and T. Inokuchi, Enhancement of the photodynamic antitumor effect by streptococcal preparation OK-432 in the mouse carcinoma, Cancer Immunol. Immunother., 2000, 49, 401–409.
N. Yamamoto and V. R. Naraparaju, A defect in inducible betagalactosidase of B lymphocytes in the osteopetrotic (mi/mi) mouse, Immunology, 1996, 88, 604–610.
W. J. Lau BH, C. L. Marsh, G. R. Barker, D. H. Koobs and R. R. Torrey, Superiority of intralesional immunotherapy with Corynebacterium parvum and Allium sativum in control of murine transitional cell carcinoma, J. Urol., 1986, 136, 701–705.
R. C. Myers, B. H. Lau, D. Y. Kunihira, R. R. Torrey, J. L. Woolley and J. Tosk, Modulation of hematoporphyrin derivative-sensitized phototherapy with corynebacterium parvum inmurine transitional cell carcinoma, Urology, 1989, 33, 230–235.
W.-C. R. Lau BH and J. Tosk, Tumor-specific T-lymphocyte cytotoxicity enhanced by low dose of C. parvum, J. Leukocyte Biol., 1987, 41, 407–411.
M. Korbelik, J. Sun and J. J. Posakony, Interaction Between Photodynamic Therapy andBCGImmunotherapyResponsible for theReduced Recurrence ofTreatedMouseTumors, Photochem. Photobiol., 2001, 73, 403–409.
H. Herr, D. Schwalb, Z. Zhang, P. Sogani, W. Fair, W. Whitmore and H. Oettgen, Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year followup of a prospective randomized trial, J. Clin. Oncol., 1995, 13, 1404–1408.
J. L. Adams and C. J. Czuprynski, Ex vivo induction of TNF-alpha and IL-6 mRNA in bovine whole blood by Mycobacterium paratuberculosis and mycobacterial cell wall components, Microb. Pathog., 1995, 19, 19–29.
M. Korbelik and I. Cecic, Enhancement of tumour response to photodynamic therapy by adjuvantmycobacterium cell-wall treatment, J. Photochem. Photobiol., B, 1998, 44, 151–158.
M. Alvaro, J. C. Nickel, D. Joseph, C. Janet and L. Ingrid Van Der, Immunotherapy of an Experimental Adenocarcinoma of the Prostate, J. Urol., 1995, 153, 1706–1710.
N. Yamamoto, Structural definition of a potent macrophage activating factor derived from vitamin D3-binding protein with adjuvant activity for antibody production, Mol. Immunol., 1996, 33, 1157–1164.
B. Z. Ngwenya and N. Yamamoto, Activation of peritoneal macrophages by lysophosphatidylcholine, Biochim. Biophys.Acta, Gen. Subj., 1985, 839, 9–15.
N. Yamamoto, J. K. Hoober and S. Yamamoto, Tumoricidal capacities of macrophages photodynamically activated with hematoporphyrin derivative, Photochem. Photobiol., 1992, 56, 245–250.
M. Korbelik, V. R. Naraparaju and N. Yamamoto, Macrophagedirected immunotherapy as adjuvant to photodynamic therapy of cancer, Br. J. Cancer, 1997, 75, 202–207.
M. Rollinghoff, A. Starzinski-Powitz, K. Pfizenmaier and H. Wagner, Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes, J. Exp. Med., 1977, 145, 455–459.
M. Glaser, Regulation of specific cell-mediated cytotoxic response against SV40-induced tumor associated antigens by depletion of suppressor T cells with cyclophosphamide in mice, J. Exp. Med., 1979, 149, 774–779.
M. J. Berendt and R. J. North, T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor, J. Exp. Med., 1980, 151, 69–80.
F. Ghiringhelli, N. Larmonier, E. Schmitt, A. Parcellier, D. Cathelin, C. Garrido, B. Chauffert, E. Solary, B. Bonnotte and F. Martin, CD4+CD25 +regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamidewhich allows immunotherapy of established tumors to be curative, Eur. J. Immunol., 2004, 34, 336–344.
M. Loeffler, J. A. Kruger and R. A. Reisfeld, Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase, Cancer Res., 2005, 65, 5027–5030.
M. E. Lutsiak, R. T. Semnani, R. De Pascalis, S. V. Kashmiri, J. Schlom and H. Sabzevari, Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide, Blood, 2005, 105, 2862–2868.
Y. Motoyoshi, K. Kaminoda, O. Saitoh, K. Hamasaki, K. Nakao, N. Ishii, Y. Nagayama and K. Eguchi, Different mechanisms for antitumor effects of low- and high-dose cyclophosphamide, Oncol. Rep., 2006, 16, 141–146.
S. Brode, T. Raine, P. Zaccone and A. Cooke, Cyclophosphamideinduced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+regulatory T cells, J. Immunol., 2006, 177, 6603–6612.
J. Taieb, N. Chaput, N. Schartz, S. Roux, S. Novault, C. Menard, F. Ghiringhelli, M. Terme, A. F. Carpentier, G. Darrasse-Jeze, F. Lemonnier and L. Zitvogel, Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines, J. Immunol., 2006, 176, 2722–2729.
A. P. Castano, P. Mroz, M. X. Wu and M. R. Hamblin, Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 5495–5500.
P. C. Kousis, B. W. Henderson, P. G. Maier and S. O. Gollnick, Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils, Cancer Res., 2007, 67, 10501–10510.
K. Kaushansky, Lineage-specific hematopoietic growth factors, N. Engl. J. Med., 2006, 354, 2034–2045.
T. H. Price, G. S. Chatta and D. C. Dale, Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans, Blood, 1996, 88, 335–340.
W. J. de Vree, M. C. Essers, H. S. de Bruijn, W. M. Star, J. F. Koster and W. Sluiter, Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo, Cancer Res., 1996, 56, 2908–2911.
H. F. Ismail, P. Fick, J. Zhang, R. G. Lynch and D. J. Berg, Depletion of neutrophils in IL-10(-/-)mice delays clearance of gastricHelicobacter infection and decreases the Th1 immune response to Helicobacter, J. Immunol., 2003, 170, 3782–3789.
J. Golab, G. Wilczynski, R. Zagozdzon, T. Stoklosa, A. Dabrowska, J. Rybczynska, M. Wasik, E. Machaj, T. Olda, K. Kozar, R. Kaminski, A. Giermasz, A. Czajka, W. Lasek, W. Feleszko and M. Jakobisiak, Potentiation of the anti-tumour effects of Photofrin-based photodynamic therapy by localized treatment with G-CSF, Br. J. Cancer, 2000, 82, 1485–1491.
D. Metcalf, Control of granulocytes and macrophages: molecular, cellular, and clinical aspects, Science, 1991, 254, 529–533.
B. Schuurman, G. Heuff, R. H. Beelen and S. Meyer, Enhanced killing capacity of human Kupffer cells after activation with human granulocyte/macrophage-colony-stimulating factor and interferon gamma, Cancer Immunol. Immunother., 1994, 39, 179–184.
G. Dranoff, E. Jaffee, A. Lazenby, P. Golumbek, H. Levitsky, K. Brose, V. Jackson, H. Hamada, D. Pardoll and R. C. Mulligan, Vaccination with irradiated tumor cells engineered to secrete murine granulocytemacrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity, Proc. Natl. Acad. Sci.U. S. A., 1993, 90, 3539–3543.
H. I. Levitsky, A. Lazenby, R. J. Hayashi and D. M. Pardoll, In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression, J. Exp. Med., 1994, 179, 1215–1224.
A. Jalili, M. Makowski, T. Switaj, D. Nowis, G. M. Wilczynski, E. Wilczek, M. Chorazy-Massalska, A. Radzikowska, W. Maslinski, L. Bialy, J. Sienko, A. Sieron, M. Adamek, G. Basak, P. Mroz, I. W. Krasnodebski, M. Jakobisiak and J. Golab, Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells, Clin. Cancer Res., 2004, 10, 4498–4508.
H. Saji, W. Song, K. Furumoto, H. Kato and E. G. Engleman, Systemic antitumor effect of intratumoral injection of dendritic cells in combination with local photodynamic therapy, Clin. Cancer Res., 2006, 12, 2568–2574.
M. Korbelik and J. Sun, Cancer treatment by photodynamic therapy combined with adoptive immunotherapy using genetically altered natural killer cell line, Int. J. Cancer, 2001, 93, 269–274.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of a themed issue on immunological aspects and drug delivery technologies in PDT.
Rights and permissions
About this article
Cite this article
Denis, T.G.S., Aziz, K., Waheed, A.A. et al. Combination approaches to potentiate immune response after photodynamic therapy for cancer. Photochem Photobiol Sci 10, 792–801 (2011). https://doi.org/10.1039/c0pp00326c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c0pp00326c