Abstract
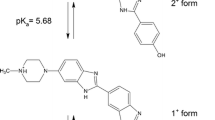
The photophysical behavior of Hoechst 33258 (H33258) in aqueous solution has been studied by steady-state and time-resolved fluorescence measurements. The intriguing intramolecular geometrical orientations of the dye bring out major modulation on its photophysical behavior, especially in the fluorescence emission characteristics with pH. It has been seen that a change in the solution pH from 7 to 4.5 enhances the emission yield by ~20 fold and this change is ~80-fold on changing the pH from 1.5 to 4.5. While a fast flipping motion among the two benzimidazole rings is considered to be one of the most probable mechanisms for the fast fluorescence decay, a more planar structure of the dicationic form at pH 4.5 having a double bond character between the two benzimidazolium groups is suggested to be the most likely fluorescent species. A similar planar structure is in fact considered to be the fluorescent emitting species of H33258 on minor groove binding to DNA. On the basis of temperature dependent fluorescence decay dynamics explored for the dye in solutions at pH 7 and 4.5, it is understood that a nearly isoenergetic double-well excited state potential is possibly involved in the excited state relaxation dynamics of the dye at pH 7. On increasing the temperature, the conversion to the planar structure is facilitated from the non-planar LE state, enhancing the emission probability of the dye.
Similar content being viewed by others
References
J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer: New York, 2006.
H. LeVine III, Quantification of beta-sheet amyloid fibril structures with thioflavin T, Methods Enzymol., 1999, 309, 274.
(a)_H. Naiki, K. Higuchi, M. Hosokawa and T. Takeda, Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavine T, Anal. Biochem., 1989, 177, 244–249
C. E. Kung and J. K. Reed, Fluorescent molecular rotors: a new class of probes for tubulin structure and assembly, Biochemistry, 1989, 28, 6678–6686.
G. Cosa, K.-S. Focsaneanu, J. R. N. McLean, J. P. McNamee and J. C. Scaiano, Photophysical properties of fluorescenct DNA-dyes bound to single and double-stranded DNA in aqueous buffered solution, Photochem. Photobiol., 2001, 73, 585–599.
A. C. Bhasikuttan, J. Mohanty, W. M. Nau and H. Pal, Efficient fluorescence enhancement and cooperative binding of an organic dye in a supra-biomolecular host-protein assembly, Angew. Chem., Int. Ed., 2007, 46, 4120–4122.
A. C. Bhasikuttan, J. Mohanty and H. Pal, Interaction of Malachite Green with Guanine-Rich Single Strand DNA: Preferential Binding to G-Quadruplex, Angew. Chem., Int. Ed., 2007, 46, 9305–9307.
S. Dutta Choudhury, J. Mohanty, H. Pal and A. C. Bhasikuttan, Cooperative Metal Ion Binding to a Cucurbit[7]uril-Thioflavin T Complex: Demonstration of a Stimulus Responsive Fluorescent Supramolecular Capsule, J. Am. Chem. Soc., 2010, 132, 1395–1401.
F. G. Loontiens, P. Regenfuss, A. Zechel, L. Dumortier and R. M. Clegg, Binding characteristics of Hoechst 33258 with calf thymus DNA, Poly[d(A-T)], and d(CCGGAATTCCGG): Multiple stoichiometries and determination of tight binding with a wide spectrum of site affinities, Biochemistry, 1990, 29, 9029–9039.
J.-H. Moon, S. K. Kim, U. Sehlstedt, A. Rodger and B. Norden, DNA structural features responsible for sequence dependent binding geometries of Hoechst 33258, Biopolymers, 1996, 38, 593–606.
L. Goracci, R. Germani, G. Savelli and D. M. Bassani, Hoechst 33258 as a pH-sensitive probe to study the interaction of amine oxide surfactants with DNA, ChemBioChem., 2005, 6, 197–203.
H. Ojha, B. M. Murari, S. Anand, M. I. Hassan, F. Ahmad and N. K. Chaudhury, Interaction of DNA minor groove binder Hoechst 33258 with Bovine Serum Albumin, Chem. Pharm. Bull., 2009, 57, 481–486.
N. V. Lyubimova, P. G. Coultas, K. Yuen and R. F. Martin, In vivo radioprotection of mouse brain endothelial cells by Hoechst 33342, Br. J. Radiol., 2001, 74, 77–82.
L. Denison, A. Haigh, G. D’Cunha and R. F. Martin, DNA Ligands as Radioprotectors: Molecular Studies with Hoechst 33342 and Hoechst 33258, Int. J. Radiat. Biol., 1992, 61, 69–74.
T. R. Downs and W. W. Wilfinger, Fluorometric quantification of DNA in cells and tissues, Anal. Biochem., 1983, 131, 538–547.
C. F. Cesarone, C. Bolognesi and L. Santi, Improved microfluorometric DNA determination in biological material using 33258 Hoechst, Anal. Biochem., 1979, 100, 188–197.
D. L. Stout and F. F. Becker, Fluorometric quantitation of single-stranded DNA: A method applicable to the technique of alkaline elution, Anal. Biochem., 1982, 127, 302–307.
M. Ladinig, W. Leupin, M. Meuwly, M. Respondek, J. Wirz and V. Zoete, Protonation equilibria of Hoechst-33258 in aqueous solution, Helv. Chim. Acta, 2005, 88, 53–67.
K. Kalninsh, D. V. Pestov and Y. K. Roshchina, Absorption and fluorescence spectra of the probe Hoechst 33258, J. Photochem. Photobiol., A, 1994, 83, 39–47.
T. Stokke and H. B. Steen, Multiple binding modes for Hoechst 33258 to DNA, J. Histochem. Cytochem., 1985, 33, 333–338.
H. Gorner, Direct and sensitized photoprocesses of bis-benzimidazole dyes and the effects of surfactants and DNA, Photochem. Photobiol., 2001, 73, 339–348.
M. Rahimian, Y. Miao and W. D. Wilson, Influence of DNA structure on adjacent site cooperative binding, J. Phys. Chem. B, 2008, 112, 8770–8778.
Y. Guan, R. Shi, X. Li, M. Zhao and Y. Li, Multiple binding modes for dicationic Hoechst 33258 to DNA, J. Phys. Chem. B, 2007, 111, 7336–7344.
A. Adhikary, V. Buschmann, C. Muller and M. Sauer, Ensemble and single-molecule fluorescence spectroscopic study of binding modes of the bis-benzimidazole derivative Hoechst 33258 with DNA, Nucleic Acids Res., 2003, 31, 2178–2186.
S. K. Pal, L. Zhao and A. H. Zewail, Water at DNA surfaces: Ultrafast dynamics in minor groove recognition, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 8113–8118.
D. Banerjee and S. K. Pal, Ultrafast charge transfer and solvation of DNA minor groove binder: Hoechst 33258 in restricted environments, Chem. Phys. Lett., 2006, 432, 257–262.
V. N. Umetskaya and Y. M. Rozanov, Mechanism of the interaction of DNA with the fluorescent dye Hoechst 33258, Biophysika, 1990, 35, 399–401.
K. E. Furse and S. A. Corcelli, The dynamics of water at DNA interfaces: computational studies of Hoechst 33258 bound to DNA, J. Am. Chem. Soc., 2008, 130, 13103–13109.
M. Shaikh, J. Mohanty, A. C. Bhasikuttan, V. D. Uzunova, W. M. Nau and H. Pal, Salt-induced Guest Relocation from a Supramolecular Cavity into a Biomolecular Pocket: Interplay between Cucurbit[7]uril and Albumin, Chem. Commun., 2008, 3681–3683.
M. J Frisch, G. W. Trucks, M. Head-Gorden, P. M. W. Gill, M. W. Wong, J. B. Foresman, B. G. Johnson, H. B. Schlegel, M. A. Robb, E. S. Replogle, R. Gomperts, J. L. Andres, K. Rahavachari, J. S. Binkley, C. Gonzalez, R. Martin, L. D. J. Fox, D. J. Defrees, J. Baker, J. J. P. Stewart, J. A. Pople, Gaussian 92, Gaussian, Inc, Pittsburgh, PA, 1992.
J. F. G. Walz, B. Terenna and D. Rolinee, Equilibrium studies on neutral red-DNA binding, Biopolymers, 1975, 14, 825–837.
J. Mohanty, A. C. Bhasikuttan, W. M. Nau and H. Pal, Host–guest complexation of neutral red with macrocyclic host molecules: Contrasting pKa shifts and binding affinities for cucurbit[7] uril and b-cyclodextrin, J. Phys. Chem. B, 2006, 110, 5132–5138.
M. Shaikh, J. Mohanty, P. K. Singh, W. M. Nau and H. Pal, Complexation of Acridine Orange by Cucurbit[7]uril and beta-cyclodextrin: Photophysical Effects and pKa Shifts, Photochem. Photobiol. Sci., 2008, 7, 408–414.
R. L. Jones and W. D. Wilson, Effect of ionic strength on the pKa of ligands bound to DNA, Biopolymers, 1981, 20, 141–154.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barooah, N., Mohanty, J., Pal, H. et al. pH and temperature dependent relaxation dynamics of Hoechst-33258: a time resolved fluorescence study. Photochem Photobiol Sci 10, 35–41 (2011). https://doi.org/10.1039/c0pp00215a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c0pp00215a