Abstract
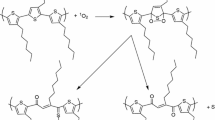
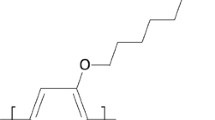
Time-dependent UV/visible absorption changes are observed on photolysis of aerated solutions of monodisperse, vinyl end-capped 2,5-diheptyloxyl substituted PV-oligomers (OPV, n = 1, 2, 3). In all cases, photolysis occurs in at least two distinct stages. Evidence for the intermediacy of singlet oxygen comes from pulse radiolysis and time-resolved luminescence studies, which confirm formation of the OPV triplet state and its quenching by molecular oxygen to produce singlet oxygen. The subsequent reaction with oligomers is observed directly by time-resolved phosphorescence and indirectly through product identification. The results strongly suggest that the reaction between singlet oxygen and OPV proceeds viacycloaddition, leading to bond scission and aldehyde group formation. The reactivity of vinylene linkages is greater than of vinyl end groups. Consequently, the photostability decreases as the chain length is extended. Implications on the oxidative degradation of poly(p-phenylenevinylene)s in optoelectronic devices, and of competing singlet oxygen and superoxideradical aniondegradation pathways, are discussed.
Similar content being viewed by others
References
J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Light emitting diodes based on conjugated polymers, Nature, 1990, 347, 539–541.
A. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices, Chem. Rev., 2009, 109, 897–1091.
S. Günes, H. Neugebauer and N. S. Sariciftci, Conjugated polymer-based organic solar cells, Chem. Rev., 2007, 107, 1324–1338.
J. Veres, S. Ogier, G. Lloyd, D. de Leeuw, Gate insulators in organic field-effect transistors, Chem. Mater., 2004, 16, 4543–4555.
S. W. Thomas, G. D. Joly and T. M. Swager, Chemical sensors based on amplifying fluorescent conjugated polymers, Chem. Rev., 2007, 107, 1339–1386.
H. Jiang, P. Taranekar, J. R. Reynolds and K. S. Schanze, Conjugated polyelectrolytes: Synthesis, photophysics and applications, Angew. Chem., Int. Ed., 2009, 48, 4300–4316.
D. Braun and A. J. Heeger, Visible light emission from semiconducting polymer diodes, Appl. Phys. Lett., 1991, 58, 1982–1984.
H. Spreitzer, H. Becker, E. Kluge, W. Kreuder, H. Schenk, R. Demandt and H. Schoo, Soluble phenyl-substituted PPVs–New materials for highly efficient polymer LEDs, Adv. Mater., 1998, 10, 1340–1343.
J. J. M. Halls, C. A. Walsh, N. C. Greenham, E. A. Marseglia, R. H. Friend, S. C. Moratti and A. B. Holmes, Efficient photodiodes from interpenetrating polymer networks, Nature, 1995, 376, 498–500.
T. Kaino, H. Kobayashi, K. Kubodera, T. Kurihara, S. Saito, T. Tsutsui and S. Tokito, Optical 3rd harmonic generation from poly(2,5-methoxy para-phenylenevinylene) thin-film, Appl. Phys. Lett., 1989, 54, 1619–1621.
G. E. Wnek, J. C. W. Chien, F. E. Karasz and C. P. Lillya, Electrically conducting derivative of poly(para-phenylene vinylene), Polymer, 1979, 20, 1441–1443.
J. L. Brédas, D. Beljonne, J. Shuai and J. M. Toussaint, Theoretical investigation of the effect of doping on the electronic properties of polyparaphenylene vinylene, Synth. Met., 1991, 43, 3743–4746.
E. Thorn-Csányi and P. Kraxner, Synthesis of soluble, all-trans poly(2,5-diheptyl-p-phenylene-vinylene) via metathesis polycondensation, Macromol. Rapid Commun., 1995, 16, 147–153.
E. Thorn-Csányi and P. Kraxner, All-trans oligomers of 2,5-dialkyl-1,4-phenylenevinylenes–metathesis preparation and characterization, Macromol. Chem. Phys., 1997, 198, 3827–3843.
R. Peetz, A. Strachota, E. Thorn-Csányi, Homologous series of 2,5-diheptyloxy-p-phenylene vinylene (DHepO-PV) oligomers with vinyl or butenyl end groups: Synthesis, isolation and microstructure, Macromol. Chem. Phys., 2003, 204, 1439–1450.
O. Narwark, S. C. J. Meskers, R. Peetz, E. Thorn-Csányi, H. Bässler, Spectroscopic characterization of p-phenylene vinylene (PV) oligomers. Part I: A homologous series of 2,5-diheptyloxy substituted PV-oligomers, Chem. Phys., 2003, 294, 1–15.
O. Narwark, A. Gerhard, S. C. J. Meskers and S. Brocke, E- Thorn-Csányi and H. Bässler, Spectroscopic characterization of p-phenylene vinylene (PV) oligomers. Part II: Selected 2,5-diheptyl substituted PV-oligomers, Chem. Phys., 2003, 294, 17–30.
J. Seixas, de Melo, J. Pina, H. D. Burrows, S. Brocke, O. Herzog, E. Thorn-Csányi, The effect of substitution and isomeric imperfection on the photophysical behaviour of p-phenylenevinylene trimers, Chem. Phys. Lett., 2004, 388, 236–241.
J. Seixas de Melo, J. Pina, H. D. Burrows, R. DiPaolo, A. L. Maçanita, Electronic Spectral and Photophysical Properties of Some p-phenylenevinylene Oligomers in Solution and Thin Films, Chem. Phys., 2006, 330, 449–456.
R. E. DiPaolo, J. Seixas, de Melo, J. Pina, H. D. Burrows, J. Morgado, A. L. Maçanita, Conformational relaxation of p-phenylenevinylene trimers in solution studied by picosecond time-resolved fluorescence, ChemPhysChem, 2007, 8, 2657–2664.
R. Wehrmann, A. Elschner and E. Thorn-Csányi, Eur. Pat. 0?964?044, A1 Anmelder: Bayer AG.
B. Tian, G. Zerbi, R. Schenk, K. Müllen, Optical-spectra and structure of oligomeric models of polyparaphenylenevinylene, J. Chem. Phys., 1991, 95, 3191–3197.
H. Meier, U. Stalmach and H. Kolshorn, Effective conjugation length and UV/vis spectra of oligomers, Acta Polym., 1997, 48, 379–384.
L. P. Candeias, J. Wildeman, G. Hadziioannou and J. M. Warman, Pulse radiolysis-optical absorption studies on the triplet states of p-phenylene vinylene oligomers in solution, J. Phys. Chem. B, 2000, 104, 8366–8371.
J. Gierschner, H.-G. Mack, L. Lüer and D. Oelkrug, Fluorescence and absorption spectra of oligophenylenevinylenes: Vibronic coupling, band shapes, and solvatochromism, J. Chem. Phys., 2002, 116, 8596–8609.
J. Gmeiner, S. Karg, M. Meier, W. Reiss, P. Strohrigl and M. Schwoerer, Synthesis, electrical conductivity and electroluminescence of poly(p-phenylene vinylene) prepared by the precursor route, Acta Polym., 1993, 44, 201–205.
F. Cacialli, R. H. Friend, S. C. Moratti and A. B. Holmes, Characterization of properties of polymeric light-emitting diodes over extended periods, Synth. Met., 1994, 67, 157–160.
R. D. Scurlock, B. J. Wang, P. R. Ogilby, J. R. Sheats and R. L. Clough, singlet oxygen as reactive intermediate in the photodegradation of an electroluminescent polymer, J. Am. Chem. Soc., 1995, 117, 10194–10202.
J. C. Scott, J. H. Kaufman, P. J. Brock, R. DiPietro, J. Salem and J. A. Goitia, Degradation and failure of MEH-PPV light-emitting diodes, J. Appl. Phys., 1996, 79, 2745–2751.
L. J. Rothberg, M. Yan, F. Papadimitrakopoulos, M. E. Galvin, E. W. Kwock and T. M. Miller, Photophysics of phenylenevinylene polymers, Synth. Met., 1996, 80, 41–58.
A. J. M. Berntsen, P. van de Weijer, Y. Croonen, C. T. H. F. Liedenbaum and J. J. M. Vleggaar, Stability of polymer light-emitting diodes, Philips J. Res., 1998, 51, 511–525.
S. M. Lipson, D. F. O’Brien, H. J. Byrne, A. P. Davey and W. J. Blau, Investigation of efficiency and photostability in polymer films, Synth. Met., 2000, 111–112, 553–557.
H. Wang, X. Tao and E. Newton, Thermal degradation kinetics and lifetime prediction of a luminescent conducting polymer, Polym. Int., 2004, 53, 20–26.
R. Pacios, A. J. Chatten, K. Kawano, J. R. Durrant, D. D. C. Bradley and J. Nelson, Efects of photo-oxidation on the performance of poly[2-methoxy-5-(3′,7′-dimethoxyoctyloxy)-1,4-phenylene vinylene]:[6,6] C-61-butyric acid methyl ester solar cells, Adv. Funct. Mater., 2006, 16, 2117–2126.
S. Chambon, A. Rivaton, J. L. Gardette and M. Firon, Durability of MDO-PPV:PCBMblends under illumination in the absence of oxygen, Sol. Energy Mater. Sol. Cells, 2008, 92, 785–792.
N. Dam, R. D. Scurlock, B. Wang, L. Ma, M. Sundahl and P. R. Ogilby, Singlet oxygen as a reactive intermediate in the photodegradation of phenylenevinylene oligomers, Chem. Mater., 1999, 11, 1302–1305.
H. D. Burrows, J. S. de Melo, C. Serpa, L. G. Arnaut, A. P. Monkman, I. Hamblett and S. Navaratnam, S1∼>T1 Intersystem Crossing in π-Conjugated Organic Polymers, J. Chem. Phys., 2001, 115, 9601–9606.
E. Thorn-Csányi, O. Narwark, R. Peetz and A. Strachota, Diheptyloxy PV oligomers in solution: Photoreactivity, Synth. Met., 1999, 101, 238.
H. W. Gibson and J. M. Pochan, Chemical modification of polymers.19. Oxidation of polyacetylene, Macromolecules, 1982, 15, 242–247.
S. Holdcroft, A photochemical study of poly(3-hexylthiophene), Macromolecules, 1991, 24, 4834–4838.
M. S. A. Abdou and S. Holdcroft, Mechanisms of photodegradation of poly(3-alkylthiophenes) in solution, Macromolecules, 1993, 26, 2954–2962.
L. Ma, X. Wang, B. Wang, J. Chen, J. Wang, K. Huang, B. Zhang, Y. Cao, Z. Han, S. Qian and S. Yao, Photooxidative degradation mechanism of model compounds of poly(p-phenylenevinylenes) [PPVs], Chem. Phys., 2002, 285, 85–94.
S. Chambon, A. Rivaton, J.-L. Gardette, M. Firon and L. Lutsen, Aging of a donor conjugated polymer: Photochemical studies of the degradation of poly[2-methoxy-5-3′,7′-dimethyloctyloxy)-1,4-phenylenevinylene], J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 317–331.
S. Chambon, A. Rivaton, J.-L. Gardette and M. Firon, Reactive intermediates in the initiation step of the photo-oxidation of MDMO-PPV, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6044–6052.
The correct IUPAC name is 1,4-bis(heptyloxy)-2,5-divinylbenzene. The name 2,5-diheptyloxy-1,4-divinylbenzene is used in order to underline the structural similarity to 2,5-diheptyloxy-p-phenylenevinylene oligomers.
R. Peetz, Ph.D. Thesis, University of Hamburg, 2000.
J. Butler, B. W. Hodgson, B. M. Hoey, E. J. Land, J. S. Lea, E. J. Lindley, F. A. P. Rushton and A. J. Swallow, Experimental studies of some moderately fast processes initiated by radiation, Radiat. Phys. Chem., 1989, 34, 633–646.
A. P. Monkman, H. D. Burrows, M. G. Miguel, I. Hamblett and S. Navaratnam, Measurement of the S0 - T1 Energy Gap in Poly(2-methoxy,5-(2′ethylhexoxy)-p-phenylenevinylene) by Triplet–triplet Energy Transfer, Chem. Phys. Lett., 1999, 307, 303–309.
A. P. Monkman, H. D. Burrows, I. Hamblett, S. Navaratnam, U. Scherf and C. Schmitt, The Triplet state of the Ladder-type Methyl-poly(p-phenylene) as Seen by Pulse Radiolysis-Energy Transfer, Chem. Phys. Lett., 2000, 327, 111–116.
A. P. Monkman, H. D. Burrows, M. da G. Miguel, I. Hamblett and S. Navaratnam, Triplet State Spectroscopy of Conjugated Polymers Studied by Pulse Radiolysis, Synth. Met., 2001, 116, 75–79.
R. Bensasson and E. J. Land, Triplet–triplet extinction coefficients via energy transfer, Trans. Faraday Soc., 1971, 67, 1904–1915.
R. V. Bensasson, E. J. Land and T. G. Truscott, Excited States and Free Radicals in Biology and Medicine, Oxford University Press, Oxford, 1993.
J. P. Keene, D. Kessel, E. J. Land, R. W. Redmond and T. G. Truscott, Direct detection of singlet oxygen sensitized by hematoporphyrinand related compounds, Photochem. Photobiol., 1986, 43, 117–120.
R. Bonnett, D. J. McGarvey, A. Harriman, E. J. Land, T. G. Truscott, U.-J. Winfield, Photophysical properties of meso-tetraphenylporphyrin and some meso(tetrahydroxyphenyl)porphyrins, Photochem. Photobiol., 1988, 48, 271–276.
S. M. Fonseca, J. Pina, L. G. Arnaut, J. Seixas, de Melo, H. D. Burrows, N. Chattopadhyay, L. Alcácer, A. Charas, J. Morgado, A. P. Monkman, U. Asawapirom, U. Scherf, R. Edge and S. Navaratnam, Triplet state and singlet oxygen formation in fluorene based alternating copolymers, J. Phys. Chem. B, 2006, 110, 8278–8283.
B.-M. Kwon, C. S. Foote and S. Khan, Chemistry of singlet oxygen. 52. Reaction with trans-stilbene, J. Org. Chem., 1989, 54, 3378–3382.
F. Wilkinson and J. G. Brummer, Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution, J. Phys. Chem. Ref. Data, 1981, 187, 809–999.
P. Bortulos, S. Dellonte, G. Beggiato and W. Corio, Interaction between singlet oxygen (1Δg) and model compounds for polymers. Flash photolytic study, Eur. Polym. J., 1977, 13, 185–188.
F. Wilkinson, W. P. Helman and A. B. Ross, Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation, J. Phys. Chem. Ref. Data, 1995, 24, 663–1021.
R. Battino (Ed.), IUPAC Solubility Data Series. Volume 7: Oxygen and Ozone, Pergamon Press, Oxford, 1981.
Y. Marcus, The Properties of Solvents, John Wiley, Chichester, 1998.
C. Schweitzer and R. Schmidt, Physical mechanisms of generation and deactivation of singlet oxygen, Chem. Rev., 2003, 103, 1685–1757.
H. Meier, H. Kretschmann, M. Lang, W. Fass, C. Albrecht, K. März, Investigations on the photoconductivity of alkoxy substituted oligo(1,4-phenyleneethynylene)s and poly(1,4-phenyleneethynylene)s, J. Prakt. Chem., 1994, 336, 297–302.
D. Beljonne, A. Ye, Z. Shuai, J.-L. Brédas, Chain length dependence of singlet and triplet exciton formation rates in organic light-emitting diodes, Adv. Funct. Mater., 2004, 14, 684–692.
A. P. Monkman, C. Rothe and S. M. King, Singlet generation yields in organic light-emitting diodes, Proc. IEEE, 2009, 97, 1597–1605.
U. Scherf and E. J. W. List, Semiconducting polyfluorenes. Towards reliable structure-property relationships, Adv. Mater., 2002, 14, 477.
S. Gamerith, H. G. Nothofer, E. J. W. List and U. Scherf, Identification of emissive interface-related defects in polyfluorene-based light emitting devices, Jpn. J. Appl. Phys., 2004, 43, L891–L893.
H. D. Burrows, M. da G. Miguel, A. P. Monkman, L. E. Horsburgh, I. Hamblett and S. Navaratnam, Pulse radiolysis studies of charge carriers in conjugated polymers, J. Chem. Phys., 2000, 112, 3082–3089.
H. D. Burrows, M. da G. Miguel, A. P. Monkman, I. Hamblett and S. Navaratnam, Transient absorption spectra of triplet states and charge carriers of conjugated polymers, J. Mol. Struct., 2001, 563–564, 41–50.
L. T. Spada and C. S. Foote, Electron-transfer photooxidation. 3. Detection of radical-ion intermediates in the cyanoaromatic-sensitized photoxidation of trans- and cis-stilbene, J. Am. Chem. Soc., 1980, 102, 391–393.
P. Wardman, Some reactions and properties of nitro radical anions important in biology and medicine, Environ. Health Perspect., 1985, 64, 309–320.
H. D. Burrows, M. E. Azenha and C. J. P. Monteiro, Homogeneous Catalysis, in Catalysis from Theory to Application, ed. J. L. Figueiredo, M. M. Pereira and J. Faria, Imprensa da Universidade de Coimbra, Coimbra, 2008, pp. 495–517.
Author information
Authors and Affiliations
Corresponding author
Additional information
† This article is published as part of a themed issue in appreciation of the many important contributions made to the field of molecular photophysics by Jan Verhoeven.
‡ Electronic supplementary information (ESI) available: Decay of transient absorption of DHepODVB-dimer triplet state at 500 nm in argon saturated and aerated solutions; mass spectrum of the photolysis product 2,5-diheptyloxy-terephthalaldehyde. See DOI: 10.1039/c0pp00053a
Rights and permissions
About this article
Cite this article
Burrows, H.D., Narwark, O., Peetz, R. et al. Mechanistic studies on the photodegradation of 2,5-dialkyloxyl-substituted para-phenylenevinylene oligomers by singlet oxygen. Photochem Photobiol Sci 9, 942–948 (2010). https://doi.org/10.1039/c0pp00053a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c0pp00053a