Abstract
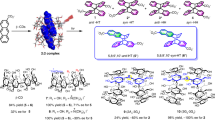
Inherently chiral molecular clip (MC) 2 binds (Z,Z)-1,3-cyclooctadiene (COD) and 1,1-diphenylpropene (DPP) in 4:1:5 THF-MeOH-H2O solution (at 25 °C) with association constants of 8800 and 27000 M-1, respectively. The thermodynamic parameters obtained from the van’t Hoff analysis (DH° = -96.4 kJ mol-1, DS° = -239 J mol-1 K-1) reveal that the binding of DPP by MC is strongly driven by the enthalpic gain from hydrophobic and p-p stacking interactions, which is however largely cancelled out by the entropic loss arising from the tight molecular association. Supramolecular photosensitization by MC 2 facilitates the Z-E isomerization of COD to chiral (E,Z)-isomer in a good E/Z ratio of 0.19 and a low ee of 0.7%, but does not appear to work with DPP probably due to the less-efficient electron transfer in the acceptor-donor-acceptor complex of DPP with MC 2.
Similar content being viewed by others
References
For reviews, see:.
H. Rau, Asymmetric photochemistry in solution, Chem. Rev., 1983, 83, 535–547.
Y. Inoue, Asymmetric photochemical reactions in solution, Chem. Rev., 1992, 92, 741–770.
J. P. Pete, Asymmetric photoreactions of conjugated enones and esters, Adv. Photochem., 1996, 21, 135–216.
S. R. L. Everitt, and Y. Inoue, in Molecular and supramolecular photochemistry, Marcel Dekker, New York, 1999.
A. G. Griesbeck and U. J. Meierhenrich, Asymmetric photochemistry and photochirogenesis, Angew. Chem. Int. Ed., 2002, 41, 3147–3154.
B. Grosch, C. N. Orlebar, E. Herdtweck, W. Massa and T. Bach, Highly enantioselective Diels-Alder reaction of a photochemically generated o-quinodimethane with olefins, Angew. Chem. Int. Ed., 2003, 42, 3693–3696.
Y. Inoue, and V. Ramamurthy, Chiral photochemistry, Marcel Dekker, New York, 2004.
Y. Inoue, Light on chirality, Nature, 2005, 436, 1099–1100.
Nobel Lectures, see
W. S. Knowles, Asymmetric hydrogenations, Angew. Chem. Int. Ed., 2002, 41, 1998–2007.
R. Noyori, Asymmetric catalysis: science and opportunities, Angew. Chem. Int. Ed., 2002, 41, 2008–2022.
K. B. Sharpless, Searching for new reactivity, Angew. Chem. Int. Ed., 2002, 41, 2024–2032.
Y. Inoue, T. Yokoyama, N. Yamasaki and A. Tai, Temperature switching of product chirality upon photosensitized enantiodifferentiating cis-trans isomerization of cyclooctene, J. Am. Chem. Soc., 1989, 111, 6480–6482.
Y. Inoue, T. Yokoyama, N. Yamasaki and A. Tai, An optical yield that increases with temperature in a photochemically induced enantiomeric isomerization, Nature, 1989, 341, 225–226.
Y. Inoue, N. Yamasaki, T. Yokoyama and A. Tai, Enantiodifferentiating Z-E photoisomerization of cyclooctene sensitized by chiral polyalkyl benzenepolycarboxylates, J. Org. Chem., 1992, 57, 1332–1345.
Y. Inoue, N. Yamasaki, T. Yokoyama and A. Tai, Highly enantiodifferentiating photoisomerization of cyclooctene by congested and/or triplex-forming chiral sensitizers, J. Org. Chem., 1993, 58, 1011–1018.
Y. Inoue, E. Matsushima and T. Wada, Pressure and temperature control of product chirality in asymmetric photochemistry. Enantiodifferentiating photoisomerization of cyclooctene sensitized by chiral benzenepolycarboxylates, J. Am. Chem. Soc., 1998, 120, 10687–10696.
M. Kaneda, S. Asaoka, H. Ikeda, T. Mori, T. Wada and Y. Inoue, Discontinuous pressure effect upon enantiodifferentiating photosensitized isomerization of cyclooctene, Chem. Commun., 2002, 1272–1273.
M. Kaneda, A. Nakamura, S. Asaoka, H. Ikeda, T. Mori, T. Wada and Y. Inoue, Pressure control of enantiodifferentiating photoisomerization of cyclooctenes sensitized by chiral benzenepolycarboxylates. The origin of discontinuous pressure dependence of the optical yield, Org. Biomol. Chem., 2003, 1, 4435–4400.
Y. Inoue, H. Ikeda, M. Kaneda, T. Sumimura, S. R. L. Everitt and T. Wada, Entropy-controlled asymmetric photochemistry: switching of product chirality by solvent, J. Am. Chem. Soc., 2000, 122, 406–407.
Y. Inoue, T. Wada, S. Asaoka, H. Sato, J.-P. Pete, Photochirogenesis: multidimensional control of asymmetric photochemistry, Chem. Commun., 2000, 251–259.
Y. Inoue, N. Sugahara and T. Wada, Vital role of entropy in photochirogenesis, Pure Appl. Chem., 2001, 73, 475–480.
T. Tamaki, T. Kokubo and K. Ichimura, Regio- and stereoselective photodimerization of anthracene derivatives included by cyclodextrins, Tetrahedron, 1987, 43, 1485–1494.
V. P. Rao and N. J. Turro, Asymmetric induction in benzoin by photolysis of benzaldehyde adsorbed in cyclodextrin cavities, Tetrahedron Lett., 1989, 30, 4641–4644.
S. Koodanjeri, A. Joy and V. Ramamurthy, Asymmetric induction with cyclodextrins: photocyclization of tropolone alkyl ethers, Tetrahedron, 2000, 56, 7003–7009.
K. Vízvárdi, K. Desmet, I. Luyten, P. Sandra, G. Hoornaert and E. V. Eycken, Asymmetric induction in intramolecular meta photocycloaddition: cyclodextrin-mediated solid-phase photochemistry of various phenoxyalkenes, Org. Lett., 2001, 3, 1173–1175.
S. Koodanjeri and V. Ramamurthy, Cyclodextrin mediated enantio and diastereoselective geometric photoisomerization of diphenylcyclopropane and its derivatives, Tetrahedron Lett., 2002, 43, 9229–9232.
J. Shailaja, S. Karthikeyan and V. Ramamurthy, Cyclodextrin mediated solvent-free enantioselective photocyclization of N-alkyl pyridones, Tetrahedron Lett., 2002, 43, 9335–9339.
H. Ikeda, T. Nihei and A. Ueno, Template-assisted stereoselective photocyclodimerization of 2-anthracenecarboxylic acid by bispyridinio-appended γ-cyclodextrin, J. Org. Chem., 2005, 70, 1237–1242.
A. Furutani, K. Katayama, Y. Ueshima, M. Ogura, Y. Tobe, H. Kurosawa, K. Tsutsumi, T. Morimoto and K. Kakiuchi, Asymmetric [2 + 2] photocycloaddition of cycloalkenone-cyclodextrin complexes to ethylene, Chirality, 2006, 18, 217–221.
Y. Inoue, F. Dong, K. Yamamoto, L.-H. Tong, H. Tsuneishi, T. Hakushi and A. Tai, Inclusion-enhanced optical yield and E/Z ratio in enantiodifferentiating photoisomerization of cyclooctene included and sensitized by ß-cyclodextrin monobenzoate, J. Am. Chem. Soc., 1995, 117, 11033–11034.
Y. Inoue, T. Wada, N. Sugahara, K. Yamamoto, K. Kimura, L.-H. Tong, X.-M. Gao, Z.-J. Hou and Y. Liu, Supramolecular photochirogenesis. 2. Enantiodifferentiating photoisomerization of cyclooctene included and sensitized by 6-O-modified cyclodextrins, J. Org. Chem., 2000, 65, 8041–8050.
Y. Gao, M. Inoue, T. Wada and Y. Inoue, Supramolecular photochirogenesis. 3. Enantiodifferentiating photoisomerization of cyclooctene included and sensitized by 6-O-mono(o-methoxybenzoyl)-ß-cyclodextrin, J. Incl. Phenom. Macrocycl. Chem., 2004, 50, 111–114.
R. Lu, C. Yang, Y. Cao, Z. Wang, T. Wada, W. Jiao, T. Mori and Y. Inoue, Supramolecular enantiodifferentiating photoisomerization of cyclooctene with modified ß-cyclodextrins: critical control by a host structure, Chem. Commun., 2008, 374–376.
A. Joy, V. Ramamurthy, J. R. Scheffer and D. R. Corbin, Enantioselective photoelectrocyclization within zeolites: tropolone methyl ether in chirally modified NaY, J. Chem. Soc., Chem. Commun., 1998, 1379–1380.
A. Joy, R. Scheffer and V. Ramamurthy, Chirally modified zeolites as reaction media: photochemistry of an achiral tropolone ether, Org. Lett., 2000, 2, 119–121.
N. J. Turro, From boiling stones to smart crystals: supramolecular and magnetic isotope control of radical-radical reactions in zeolites, Acc. Chem. Res., 2000, 33, 637–646.
T. Wada, M. Shikimi, Y. Inoue, G. Lem and N. J. Turro, First photosensitized enantiodifferentiating isomerization by optically active sensitizer immobilized in zeolite supercages, Chem. Commun., 2001, 1864–1865.
K. C. W. Chong, J. Sivaguru, T. Shichi, Y. Yoshimi, V. Ramamurthy and J. R. Scheffer, Use of chirally modified zeolites and crystals in photochemical asymmetric synthesis, J. Am. Chem. Soc., 2002, 124, 2858–2859.
J. Sivaguru, A. Natarajan, L. S. Kaanumalle, J. Shailaja, S. Uppili, A. Joy and V. Ramamurthy, Asymmetric photoreactions within zeolites: role of confinement and alkali metal ions, Acc. Chem. Res., 2003, 36, 509–521.
J. Sivaguru, T. Poon, R. Franz, S. Jockusch, W. Adam and N. J. Turro, Stereocontrol within confined spaces: enantioselective photooxidation of enecarbamates inside zeolites supercages, J. Am. Chem. Soc., 2004, 126, 10816–10817.
J. Sivaguru, H. Saito, M. R. Solomon, L. S. Kaanumalle, T. Poon, S. Jockusch, W. Adam, V. Ramamurthy, Y. Inoue and N. J. Turro, Control of chirality by cations in confined spaces: photooxidation of enecarbamates inside zeolite supercages, Photochem. Photobiol., 2006, 82, 123–131.
K. Sivasubramanian, L. S. Kaanumalle, S. Uppili and V. Ramamurthy, Value of zeolites in asymmetric induction during photocyclization of pyridones, cyclohexadienones and naphthalenones, Org. Biomol. Chem., 2007, 5, 1569–1576.
G. Fukuhara, T. Mori, T. Wada and Y. Inoue, Entropy-controlled supramolecular photochirogenesis: enantiodifferentiating Z-E photoisomerization of cyclooctene included and sensitized by permethylated 6-O-benzoyl-ß-cyclodextrin, Chem. Commun., 2005, 4199–4201.
C. Yang, A. Nakamura, G. Fukuhara, Y. Origane, T. Mori, T. Wada and Y. Inoue, Pressure and temperature-controlled enantiodifferentiating [4 + 4] photocyclodimerization of 2-anthracenecarboxylate mediated by secondary face- and skeleton-modified γ-cyclodextrins, J. Org. Chem., 2006, 71, 3126–3136.
G. Fukuhara, T. Mori, T. Wada and Y. Inoue, The first supramolecular photosensitization of enantiodifferentiating bimolecular reaction: anti-Markovnikov photoaddition of methanol to 1,1-diphenylpropene sensitized by modified ß-cyclodextrin, Chem. Commun., 2006, 1712–1714.
G. Fukuhara, T. Mori, T. Wada and Y. Inoue, Entropy-controlled supramolecular photochirogenesis: enantiodifferentiating Z-E photoisomerization of cyclooctene included and sensitized by permethylated 6-O-modified ß-cyclodextrins, J. Org. Chem., 2006, 71, 8233–8243.
C. Yang, T. Mori, T. Wada and Y. Inoue, Supramolecular enantiodifferentiating photoisomerization of (Z,Z)-1,3-cyclooctadiene included and sensitized by naphthalene-modified cyclodextrins, New J. Chem., 2007, 31, 697–702.
G. Fukuhara, S. Madenci, J. Polkowska, F. Bastkowski, F.-G. Klärner, Y. Origane, M. Kaneda, T. Mori, T. Wada and Y. Inoue, Inherently chiral molecular clips: synthesis, chiroptical properties, and application to chiral discrimination, Chem. Eur. J., 2007, 13, 2473–2479.
Other type of chiral MCs, see:.
C. S. Wilcox, Tröger’s base analogs. New structural units for the preparation of chiral hosts and metal ligands, Tetrahedron Lett., 1985, 26, 5749–5752.
C. S. Wilcox, L. M. Greer and V. Lynch, Synthesis of chiral molecular clefts. New armatures for biomimetic systems, J. Am. Chem. Soc., 1987, 109, 1865–1867.
M. Harmata and T. Murray, Molecular clefts. 1. Synthetic methodology for the preparation of analogues of Kagan’s ether, J. Org. Chem., 1989, 54, 3761–3763.
P. P. Castro, T. M. Georgiadis and F. Diederich, Chiral recognition in clefts and cyclophane cavities shaped by the 1,1’-binaphthyl major groove, J. Org. Chem., 1989, 54, 5835–5838.
M. Harmata and C. L. Barnes, Molecular clefts 2. An analogue of Kagan’s ether as a molecular cleft: synthesis and clathrate formation with ethyl acetate, Tetrahedron Lett., 1990, 31, 1825–1828.
M. Harmata and C. L. Barnes, Molecular clefts. 3. The crystal structure of a chiral molecular tweezer and its guest, J. Am. Chem. Soc., 1990, 112, 5655–5657.
V. A. Montero, L. Tomlinson, K. N. Houk and F. Diederich, Selective α,ω-dicarboxylic acid recognition in a chiral cleft shaped by the 9,9’-spirobifluorene unit, Tetrahedron Lett., 1991, 32, 5309–5312.
E. Martinborough, T. M. Denti, P. P. Castro, T. B. Wyman, C. B. Knobler and F. Diederich, Chiral 1,1’-binaphthyl molecular clefts for the complexation of excitatory amino-acid derivatives, Helv. Chim. Acta, 1995, 78, 1037–1066.
L. J. D’Souza and U. Maitra, Design, synthesis, and evaluation of bile acid-based molecular tweezers, J. Org. Chem., 1996, 61, 9494–9502.
J. Fleischhauer, M. Harmata, M. Kahraman, A. Koslowski and C. J. Welch, The determination of the absolute configuration of a chiral molecular tweezer using CD spectroscopy, Tetrahedron Lett., 1997, 38, 8655–8658.
U. Maitra, P. Rao, V. Kurmar, P. R. Balasubramanian and L. Mathew, Solvent effect in molecular recognition: determining binding constants in different solvents following an extraction based protocol, Tetrahedron Lett., 1998, 39, 3255–3258.
M. C. Kimber, A. C. Try, L. Painter, M. M. Harding and P. Turner, Synthesis of functionalized chiral carbocyclic cleft molecules complementary to Tröger’s base derivatives, J. Org. Chem., 2000, 65, 3042–3046.
V. K. Potluri and U. Maitra, Bile acid-derived molecular tweezers: study of solvent effects in binding, and determination of thermodynamic parameters by an extraction-based protocol, J. Org. Chem., 2000, 65, 7764–7769.
C. Pardo, E. Sesmilo, E. Gutiérrez-Puebla, A. Monge, J. Elguero and A. Fruchier, New chiral molecular tweezers with a bis-Tröger’s base skeleton, J. Org. Chem., 2001, 66, 1607–1611.
R. A. Pascal, Jr., M. S. Mathai, X. Shen and D. M. Ho, Trimerization of a steroid ketone to form a chiral molecular cleft, Angew. Chem. Int. Ed., 2001, 40, 4746–4748.
C.-P. Du, J.-S. You, X.-Q. Yu, C.-L. Liu, J.-B. Lan, R.-G. Xie, Homochiral molecular tweezers as hosts for the highly enantioselective recognition of amino acid derivatives, Tetrahedron: Asymmetry, 2003, 14, 3651–3656.
T. Mas, C. Pardo, F. Salort, J. Elguero and M. R. Torres, A new entry to bis-Tröger’s bases, Eur. J. Org. Chem., 2004, 1097–1104.
M. Harmata, Chiral molecular tweezers, Acc. Chem. Res., 2004, 37, 862–873.
T. Mas, C. Pardo and J. Elguero, Use of Tröger’s base as a scaffold for new chiral molecular tweezers: synthesis of trimeric, fused Tröger’s bases, Helv. Chim. Acta, 2005, 88, 1199–1207.
T. Ema, N. Ouchi, T. Doi, T. Korenaga and T. Sakai, Highly sensitive chiral shift reagent bearing two zinc porphyrins, Org. Lett., 2005, 7, 3985–3988.
J. Artacho, P. Nilsson, K.-E. Bergquist, O. F. Wendt, K. Wärnmark, The synthesis and characterization of all diastereomers of a linear symmetrically fused tris-Tröger’s base analogue: new chiral cleft compounds, Chem. Eur. J., 2006, 12, 2692–2701.
C. K. Y. Lee, J. L. Groneman, P. Turner, L. M. Rendina and M. M. Harding, Synthesis and X-ray crystallographic analysis of chiral pyridyl substituted carbocyclic molecular clefts, Tetrahedron, 2006, 62, 4870–4878.
M. Harmata, N. L. Calkins, R. G. Baughman and C. L. Barnes, New chiral benzothiazine ligand and its use in the synthesis of a chiral receptor, J. Org. Chem., 2006, 71, 3650–3652.
M. Palomino-Schätzlein, A. Virgili, S. Gil and C. Jaime, Di-(R,R)-1-[10-(1-hydroxy-2,2,2-trifluoroethyl)-9-anthryl]-2,2,2-trifluoroethyl muconate: a highly chiral cavity for enantiodiscrimination by NMR, J. Org. Chem., 2006, 71, 8114–8120.
Y. Chen, N. She, X. Meng, G. Yin, A. Wu and L. Isaacs, Chiral molecular clips control orthogonal crystalline organization, Org. Lett., 2007, 9, 1899–1902.
A. Friberg, C. Olsson, F. Ek, U. Berg and T. Frejd, Cleft molecules as organocatalysts in an asymmetric hetero-Diels-Alder reaction, Tetrahedron: Asymmetry, 2007, 18, 885–891.
For (Z,Z)-1,3-cyclooctadiene, see:.
Y. Inoue, H. Tsuneishi, T. Hakushi and A. Tai, Optically active (E,Z)-1,3-cyclooctadiene: first enantioselective synthesis through asymmetric photosensitization and chiroptical property, J. Am. Chem. Soc., 1997, 119, 472–478.
M. Shi and Y. Inoue, Enantiodifferentiating photoisomerization of (Z)-cyclooctene and (Z,Z)-cycloocta-1,3-diene sensitized by chiral aromatic amides, J. Chem. Soc., Perkin Trans. 2, 1998, 1725–1729.
For 1,1-diphenylpropene, see:.
S. Asaoka, T. Kitazawa, T. Wada and Y. Inoue, Enantiodifferentiating anti-Markovnikov photoaddition of alcohols to 1,1-diphenylalkenes sensitized by chiral naphthalenecarboxylates, J. Am. Chem. Soc., 1999, 121, 8486–8498.
S. Asaoka, T Wada and Y. Inoue, Microenvironmental polarity control of electron-transfer photochirogenesis. Enantiodifferentiating polar addition of 1,1-diphenyl-1-alkenes photosensitized by saccharide naphthalenecarboxylates, J. Am. Chem. Soc., 2003, 125, 3008–3027.
C. Jasper, T. Schrader, J. Panitzky, F.-G. Klärner, Selective complexation of N-alkylpyridinium salts: recognition of NAD+ in water, Angew. Chem. Int. Ed., 2002, 41, 1355–1358.
M. Fokkens, C. Jasper, T. Schrader, F. Koziol, C. Ochsenfeld, J. Polkowska, M. Lobert, B. Kahlert, F.-G. Klärner, Selective complexation of N-alkylpyridinium salts: binding of NAD+ in water, Chem. Eur. J., 2005, 11, 477–494.
M. Fokkens, T. Schrader, F.-G. Klärner, A molecular tweezer for lysine and arginine, J. Am. Chem. Soc., 2005, 127, 14415–14421.
F.-G. Klärner, B. Kahlert, A. Nellesen, J. Zienau, C. Ochsenfeld and T. Schrader, Molecular tweezer and clip in aqueous solution: unexpected self-assembly, powerful host-guest complex formation, quantum chemical 1H NMR shift calculation, J. Am. Chem. Soc., 2006, 128, 4831–4841.
P. Talbiersky, F. Bastkowski, F.-G. Klärner and T. Schrader, Molecular clip and tweezer introduce new mechanisms of enzyme inhibition, J. Am. Chem. Soc., 2008, 130, 9824–9828.
N. M. Green, Thermodynamics of the binding of biotin and some analogues by avidin, Biochem. J., 1966, 101., 774–780.
W. S. Jeon, K. Moon, S. H. Park, H. Chun, Y. H. Ko, J. Y. Lee, E. S. Lee, S. Samal, N. Selvapalam, M. V. Rekharsky, V. Sindelar, D. Sobransingh, Y. Inoue, A. E. Kaifer and K. Kim, Complexation of ferrocene derivatives by the cucurbit[7]uril host: a comparative study of the cucurbituril and cyclodextrin host families, J. Am. Chem. Soc., 2005, 127, 12984–12989.
M. V. Rekharsky, T. Mori, C. Yang, Y. H. Ko, N. Selvapalam, H. Kim, D. Sobransingh, A. E. Kaifer, S. Liu, L. Isaacs, W. Chen, S. Moghaddam, M. K. Gilson, K. Kim and Y. Inoue, A synthetic host-guest system achieves avidin-biotin affinity by overcoming enthalpy-entropy compensation, Proc. Natl. Acad. Sci. USA, 2007, 104, 20737–20742.
These thermodynamic parameters are considerably large compared with those of typical cyclodextrin complexations that Rekharsky and Inoue previously demonstrated; see:.M. V. Rekharsky and Y. Inoue, Complexation thermodynamics of cyclodextrins, Chem. Rev., 1998, 98, 1875–1917.
S. L. Murov, Handbook of photochemistry, Marcel Dekker, New York, 1973.
T. Majima, C. Pac and H. Sakurai, Photochemical reactions of aromatic compounds. XXXI. Exciplex, quenching by pyridine, methylated pyridines, and methylated imidazoles and termolecular interaction in the excited singlet state, Bull. Chem. Soc. Jpn., 1978, 51, 1811–1817.
R. A. Caldwell, D. Creed and H. Ohta, Exciplex quenching. Geometric and electronic requirements, J. Am. Chem. Soc., 1975, 97, 3246–3247.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This paper was published as part of the themed issue in honour of Nicholas Turro.
Current address: Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Katahira, Aoba-ku, Sendai, 980-8577, Japan.
Rights and permissions
About this article
Cite this article
Fukuhara, G., Klärner, FG., Mori, T. et al. Supramolecular complexation and photochirogenesis with inherently chiral molecular clip: enantiodifferentiating photoisomerization of (Z,Z)-1,3-cyclooctadiene and polar photoaddition to 1,1-diphenylpropene. Photochem Photobiol Sci 7, 1493–1500 (2008). https://doi.org/10.1039/b812186a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b812186a