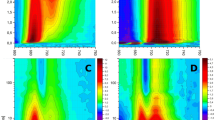
Abstract
Nanosecond transient absorption spectroscopy has been used to study reaction centre (RC) chlorophyll triplet quenching by carotenoid in intact photosystem II cores from T. elongatus with closed RCs. We found a triplet β-carotene (3Car) signal (absorption difference maximum at 530 nm) that is sensitized by the RC chlorophyll (Chl) triplet with a formation time of ca. 190 ns, has a decay time of 7 μs and is formed with a quantum yield between 10 and 20%. The 3Car signal is assigned to the β-carotene on the D2 branch of the RC. We thus propose a new photoprotection mechanism operative in closed RCs where—as a consequence of the negative charge on the quinone QA—the triplet chlorophyll (3Chl) is formed by the radical pair (RP) mechanism on the normally inactive D2 branch where it can be subsequently quenched by the D2β-carotene. We suggest that the D2 branch becomes active when the RCs are closed under high light fluence conditions. Under these conditions the D2 branch plays a photoprotective role. This interpretation allows combining many seemingly inconsistent observations in the literature and reveals the so far missing RC triplet quenching mechanism in photosystem II. The newly proposed mechanism also explains the reason why this RC triplet quenching is not observed in isolated D1-D2-cyt b559 RCs. If QA is either not present at all (as in the isolated RC) or is not charged (as in open RCs or with doubly reduced QA) then the RC 3Chl is formed on the D1 branch. The D1 branch 3Chl can not be quenched due to the large distance to the β-carotene. This interpretation is actually in line with the well-known 3RC quenching mechanism in bacterial RCs, where also the carotenoid in the (analogous to the D2 branch) B-branch of the RC becomes the quencher.
Similar content being viewed by others
References
A. Telfer, Too much light? How beta-carotene protects the photosystem II reaction centre, Photochem. Photobiol. Sci., 2005, 4, 950–956.
B. Demmig-Adams, A. M. Gilmore and W. W. Adams, Carotenoids. 3. In vivo functions of carotenoids in higher plants, FASEB J., 1996, 10, 403–412.
H. A. Frank, Incorporation of carotenoids into reaction center and light-harvesting pigments-protein complexes, in The Photochemistry of Carotenoids, ed. H. A. Frank, A. J. Young, G. Britton and R. J. Cogdell, Kluwer Academic Publishers, Dordrecht, 1999pp. 235–244.
H. Paulsen, Carotenoids and the assembly of light-harvesting complexes, in The Photochemistry of Carotenoids, ed. H. A. Frank, A. J. Young, G. Britton and R. J. Cogdell, Kluwer Academic Publishers, Dordrecht, 1999pp. 123–135.
A. Zouni, H. T. Witt, J. Kern, P. Fromme, N. Krauss, W. Saenger and P. Orth, Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution, Nature, 2001, 409, 739–743.
N. Kamiya, J.-R. Shen, Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7 Å resolution, Proc. Natl. Acad. Sci. USA, 2003, 100, 98–103.
K. N. Ferreira, T. M. Iverson, K. Maghlaoui, J. Barber and S. Iwata, Architecture of the photosynthetic oxygen-evolving center, Science, 2004, 303, 1831–1838.
J. Biesiadka, B. Loll, J. Kern, K.-D. Irrgang and A. Zouni, Crystal structure of cyanobacterial photosystem II at 3.2 Å resolution: a closer look at the Mn-cluster, Phys. Chem. Chem. Phys., 2004, 6, 4733–4736.
B. Loll, J. Kern, W. Saenger, A. Zouni and J. Biesiadka, Towards complete cofactor arrangement in the 3.0 angstrom resolution structure of photosystem II, Nature, 2005, 438, 1040–1044.
R. J. Cogdell and H. A. Frank, How carotenoids function in photosynthetic bacteria, Biochim. Biophys. Acta, 1987, 895, 63–79.
K. R. Naqvi, T. B. Melo, B. B. Raju, T. Javorfi, I. Simidjiev and G. Garab, Quenching of chlorophyll a singlets and triplets by carotenoids in light-harvesting complex of photosystem II: Comparison of aggregates with trimers, Spectrochim. Acta, Part A, 1997, 53, 2659–2667.
A. A. Krasnovsky, Delayed fluorescence and phosphorescence of plant pigments, Photochem. Photobiol., 1982, 36, 733–741.
H. A. Frank and R. J. Cogdell, Carotenoids in photosynthesis, Photochem. Photobiol., 1996, 63, 257–264.
H. A. Frank and C. A. Violette, Monomeric bacteriochlorophyll is required for the triplet energy transfer between the primary donor and the carotenoid in photosynthetic bacterial reaction centers, Biochim. Biophys. Acta, 1989, 976, 222–232.
R. J. Cogdell, T. D. Howard, R. Bittl, E. Schlodder, I. Geisenheimer and W. Lubitz, How carotenoids protect bacterial photosynthesis, Philos. Trans. R. Soc. London, Ser. B, 2000, 355, 1345–1349.
D. L. Dexter, A theory of sensitized luminescence in solids, J. Chem. Phys., 1953, 21, 836–850.
A. Telfer, S. Dhami, S. M. Bishop, D. Phillips and J. Barber, β-carotene quenches singlet oxygen formed by isolated photosystem II reaction centers, Biochemistry, 1994, 33, 14469–14474.
C. A. Tracewell, Secrets of carotenoid binding, Structure, 2004, 12, 733–734.
A. Telfer, T. A. Moore, S. Styring, A. W. Rutherford, P. Fromme, E. M. Aro and G. W. Brudvig, What is β-carotene doing in the photosystem II reaction centre?, Philos. Trans. R. Soc. London, Ser. B, 2002, 357, 1431–1440.
H. Ishikita, B. Loll, J. Biesiadka, J. Kern, K.-D. Irrgang, A. Zouni, W. Saenger, E.-W. Knapp, Function of two β-carotenes near the D1 and D2 proteins in photosystem II dimers, Biochim. Biophys. Acta, 2007, 1767, 79–87.
J. De Las Rivas, A. Telfer and J. Barber, 2-Coupled beta-carotene molecules protect P680 from photodamage in isolated Photosystem-II reaction centres, Biochim. Biophys. Acta, 1993, 1142, 155–164.
P. Faller, A. Pascal and A. W. Rutherford, beta-carotene redox reactions in photosystem II: Electron transfer pathway, Biochemistry, 2001, 40, 6431–6440.
Y. Deligiannakis, J. Hanley and A. W. Rutherford, Carotenoid oxidation in photosystem II: 1D- and 2D-electron spin-echo envelope modulation study, J. Am. Chem. Soc., 2000, 122, 400–401.
J. Hanley, Y. Deligiannakis, A. Pascal, P. Faller and A. W. Rutherford, Carotenoid oxidation in photosystem II, Biochemistry, 1999, 38, 8189–8195.
A. Telfer, T. C. Oldham, D. Phillips and J. Barber, Singlet oxygen formation detected by near-infrared emission from isolated photosystem II reaction centres: Direct correlation between P680 triplet decay and luminescence rise kinetics and its consequences for photoinhibition, J. Photochem. Photobiol., B, 1999, 48, 89–96.
P. Horton and A. V. Ruban, Molecular design of the photosystem II light-harvesting antenna: Photosynthesis and photoprotection, J. Exp. Bot., 2005, 56, 365–373.
N. E. Holt, G. R. Fleming and K. K. Niyogi, Toward an understanding of the mechanism of nonphotochemical quenching in green plants, Biochemistry, 2004, 43, 8281–8289.
A. R. Holzwarth, M. G. Müller, M. Reus, M. Nowaczyk, J. Sander, M. Rögner, Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: Pheophytin is the primary electron acceptor, Proc. Natl. Acad. Sci. USA, 2006, 103, 6895–6900.
A. J. Hoff, H. Rademaker, R. van Grondelle and L. N. M. Duysens, On the magnetic field dependence of the yield of the triplet state in reaction centers of photosynthetic bacteria, Biochim. Biophys. Acta, 1977, 460, 547–554.
Y. Takahashi, Ö. Hansson, P. Mathis and K. Satoh, Primary radical pair in the photosystem II reaction centre, Biochim. Biophys. Acta, 1987, 893, 49–59.
M.-L. Groot, E. J. G. Peterman, P. J. M. van Kan, I. H. M. van Stokkum, J. P. Dekker and R. van Grondelle, Temperature-dependent triplet and fluorescence quantum yields of the photosystem II reaction center described in a thermodynamic model, Biophys. J., 1994, 67, 318–330.
J. R. Durrant, L. B. Giorgi, J. Barber, D. R. Klug and G. Porter, Characterisation of triplet states in isolated photosystem II reaction centers: Oxygen quenching as a mechanism for photodamage, Biochim. Biophys. Acta, 1990, 1017, 167–175.
I. Yruela, M. S. Churio, T. Gensch, S. E. Braslavsky and A. R. Holzwarth, Optoacoustic and singlet oxygen near-IR emission study of the isolated D1-D2-cyt b-559 reaction center complex of photosystem II. Protein, movement associated with charge separation, J. Phys. Chem., 1994, 98, 12789–12795.
A. Kamlowski, L. Frankemöller, A. J. Van Der Est, D. Stehlik and A. R. Holzwarth, Evidence for delocalization of the triplet state 3P680 in the D1D2cyt b559-complex of photosystem II, Ber. Bunsen-Ges. Phys. Chem., 1996, 100, 2045–2051.
F. J. E. Van Mieghem, K. Satoh and A. W. Rutherford, A chlorophyll tilted 30° relative to the membrane in the photosystem II reaction centre, Biochim. Biophys. Acta, 1991, 1058, 379–385.
T. Noguchi, Dual role of triplet localization on the accessory chlorophyll in the photosystem II reaction center: Photoprotection and photodamage of the D1 protein, Plant Cell Physiol., 2002, 43, 1112–1116.
M. Seibert, R. Picorel, A. B. Rubin and J. S. Connolly, Spectral, photophysical, and stability properties of isolated photosystem II reaction center, Plant Physiol., 1988, 87, 303–306.
F. van Mieghem, K. Brettel, B. Hillmann, A. Kamlowski, A. W. Rutherford and E. Schlodder, Charge recombination reactions in photosystem II. 1. Yields, recombination pathways, and kinetics of the primary pair, Biochemistry, 1995, 34, 4798–4813.
B. Liu, A. Napiwotzki, H. -J. Eckert, H.-J. Eichler and G. Renger, Studies on the recombination kinetics of the radical pair P680+Pheo− in isolated PS II core complexes from spinach, Biochim. Biophys. Acta, 1993, 1142, 129–138.
B. Hillmann, K. Brettel, F. van Mieghem, A. Kamlowski, A. W. Rutherford and E. Schlodder, Charge recombination reactions in photosystem II. 2. Transient absorbance difference spectra and their temperature dependence, Biochemistry, 1995, 34, 4814–4827.
Y. Deligiannakis and A. W. Rutherford, Reaction centre photochemistry in cyanide-treated photosystem II, Biochim. Biophys. Acta, 1998, 1365, 354–362.
W. O. Feikema, P. Gast, I. B. Klenina and I. I. Proskuryakov, EPR characterisation of the triplet state in photosystem II reaction centers with singly reduced primary acceptor QA, Biochim. Biophys. Acta, 2005, 1709, 105–112.
M. K. Bosch, I. I. Proskuryakov, P. Gast and A. J. Hoff, Time-resolved EPR study of the primary donor triplet in D1-D2-cyt b559 complexes of photosystem II: Temperature dependence of spin-lattice relaxation, J. Phys. Chem., 1996, 100, 2384–2390.
S. Santabarbara, E. Bordignon, R. C. Jennings and D. Carbonera, Chlorophyll triplet states associated with photosystem II of thylakoids, Biochemistry, 2002, 41, 8184–8194.
S. Santabarbara, R. C. Jennings, D Carbonera, Analysis of photosystem II triplet states in thylakoids by fluorescence detected magnetic resonance in relation to the redox state of the primary quinone acceptor QA, Chem. Phys., 2003, 294, 257–266.
H. Kuhl, J. Kruip, A. Seidler, A. Krieger-Liszkay, M. Bunker, D. Bald, A. J. Scheidig, M. Rögner, Towards structural determination of the water-splitting enzyme - Purification, crystallization, and preliminary crystallographic studies of photosystem II from a thermophilic cyanobacterium, J. Biol. Chem., 2000, 275, 20652–20659.
P. J. van Leeuwen, M. C. Nieveen, E. J. van de Meent, J. P. Dekker, H. J. van Gorkom, Rapid and simple isolation of pure photosystem II core and reaction center particles from spinach, Photosynth. Res., 1991, 28, 149–153.
P. Schmidt, T. Gensch, A. Remberg, W. Gärtner, S. E. Braslavsky and K. Schaffner, The complexity of the Pr to Pfr phototransformation kinetics is an intrinsic property of native phytochrome, Photochem. Photobiol., 1998, 68, 754–761.
M. -L. Groot, E. J. G. Peterman, I. H. M. van Stokkum, J. P. Dekker, R. van Grondelle, Triplet and fluorescing states of the CP47 antenna complex of photosystem II studied as a function of temperature, Biophys. J., 1995, 68, 281–290.
P. Mathis, W. L. Butler and K. Satoh, Carotenoid triplet state and chlorophyll fluorescence quenching in chloroplasts and sub chloroplasts particles, Photochem. Photobiol., 1979, 30, 603–614.
E. Schlodder and K. Brettel, Primary charge separation in closed photosystem II with a lifetime of 11 ns. Flash-absorption spectroscopy with O2-evolving photosystem II complexes from Synechococcus, Biochim. Biophys. Acta, 1988, 933, 22–34.
E. J. G. Peterman, F. M. Dukker, R. van Grondelle and H. van Amerongen, Chlorophyll a and carotenoid triplet states in light-harvesting complex II of higher plants, Biophys. J., 1995, 69, 2670–2678.
S. S. Lampoura, V. Barzda, G. M. Owen, A. J. Hoff and H. van Amerongen, Aggregation of LHCII leads to a redistribution of the triplets over the central xanthophylls in LHCII, Biochemistry, 2002, 41, 9139–9144.
R. J. Porra, W. A. Thompson and P. E. Kriedemann, Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy, Biochim. Biophys. Acta, 1989, 975, 384–394.
G. H. Schatz, H. Brock and A. R. Holzwarth, Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density, Proc. Natl. Acad. Sci. USA, 1987, 84, 8414–8418.
M. Szczepaniak, J. Sander, M. Nowaczyk, M. G. Müller, M. Rögner and A. R. Holzwarth, Charge separation, stabilization, and protein relaxation in photosystem II core particles with closed reaction center, Biophys. J., 2008, in press.
G. H. Schatz, H. Brock and A. R. Holzwarth, A kinetic and energetic model for the primary processes in photosystem II, Biophys. J., 1988, 54, 397–405.
A. Ogrodnik, M. Volk, and M. E. Michel-Beyerle, On the energetics of the states 1P,3P and P+H− in reaction centers of Rb sphaeroides, in The Photosynthetic Bacterial Reaction Center. 149, ed. J. Breton and A. Vermeglio, Plenum Press, New York, 1988, pp. 177–183.
M. E. Michel-Beyerle, H. Scheer, H. Seidlitz, D. Tempus and R. Haberkorn, Time-resolved magnetic field effect on triplet formation in photosynthetic reaction centers of Rhodopseudomonas sphaeroides R-26, FEBS Lett., 1979, 100, 9–12.
M. Kammel, J. Kern, W. Lubitz and R. Bittl, Photosystem II single crystals studied by transient EPR: The light-induced triplet state, Biochim. Biophys. Acta, 2003, 1605, 47–54.
Z. Liu, H. Yan, K. Wang, T. Kuang, J. Zhang, L. Gui, X. An and W. Chang, Crystal structure of spinach major light-harvesting complex at 2.72 angstrom resolution, Nature, 2004, 428, 287–292.
R. Croce, M. Mozzo, T. Morosinotto, A. Romeo, R. Hienerwadel, R Bassi, Singlet and triplet state transitions of carotenoids in the antenna complexes of higher-plant photosystem I, Biochemistry, 2007, 46, 3846–3855.
Z. Q. You, C. P. Hsu and G. R. Fleming, Triplet-triplet energy-transfer coupling: Theory and calculation, J. Chem. Phys., 2006, 124, 044506–1–044506–10.
S. Santabarbara, G. Agostini, P. Heathcote and D. Carbonera, A fluorescence detected magnetic resonance investigation of the carotenoid triplet states associated with photosystem II of isolated spinach thylakoid membranes, Photosynth. Res., 2005, 86, 283–296.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Martínez-Junza, V., Szczepaniak, M., Braslavsky, S.E. et al. A photoprotection mechanism involving the D2 branch in photosystem II cores with closed reaction centers. Photochem Photobiol Sci 7, 1337–1343 (2008). https://doi.org/10.1039/b809884k
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b809884k