Abstract
It is important to understand what environmental parameters may regulate microcystin (MC) production and congener type. To determine if environmental conditions in two hydraulically connected lakes can influence MC production and congener ratios, we incubated dialysis bags containing phytoplankton from mesotrophic/eutrophic Muskegon Lake into hypereutrophic Bear Lake (Michigan, USA) and vice versa. Strong cyanobacteria growth was observed in all dialysis bags with Bear Lake phytoplankton in July and August. Phytoplankton communities were dominated by Aphanizomenon aphanizomenoides, Microcystis wesenbergii, Limnothrix redekei. MC concentrations were correlated with M. wesenbergii and A. aphanizomenoides biovolume. MC concentrations in bags incubated in the Muskegon Lake with Bear Lake water were significantly higher than the other bags. The higher light intensity and total nitrogen concentration may have caused the increase of MC production. The MC-LR/MC-RR ratios varied with sample origin but not with lake of incubation, indicating that physical environmental factors (water temperature and turbidity) were not the reasons for different toxin production ratios. Differences in total phosphorus concentrations might be one reason for the dissimilarity of the MC-LR/MC-RR ratio between the two lakes. The higher light intensity and NO3-N concentration in Muskegon Lake are two factors contributing to an increase of MC production.
Similar content being viewed by others
Introduction
Toxic cyanobacterial blooms occur frequently in eutrophic fresh waters worldwide1. Recent increases in cyanobacterial blooms are a cause for concern because they are known to produce a wide variety of toxins. Cyanotoxins can threaten the supply of drinking water and fisheries-related food supplies2,3. In addition, the toxins can accumulate in organisms and be transferred via aquatic food webs, presenting potential risks to human health4. The most widespread cyanotoxins in the environment is microcystin (MC), and more than 100 MC congeners have been identified from cyanobacterial blooms and cultures5. Congener type is a very important consideration in a bloom because the dominance of one congener over another will affect the toxicity6. Microcystin-LR (MC-LR) is the most common congener in freshwater7, also is one of the most hepatotoxic congeners8. Mouse assays indicated that the MC-LR and MC-LA variants were equally toxic, but were 12 times more toxic than another common congener MC-RR. Water bodies with regular dominance of specific taxa are likely to exhibit characteristic patterns of microcystin variants9. Many studies have focused on environmental parameters, such as water temperature10,11, phosphorus and nitrogen12, stoichiometric ratio of available nitrogen to phosphorus13, and pH14 on total microcystin production. However, only a few studies evaluated the effect of environmental parameters on the ratio of MC congeners and their relative abundances. In Anabaena 90, Rapala et al.15 suggested that the different MC variants were affected by temperature. In Microcystis aeruginosa HUB 5-2-4, Hesse and Kohl16 indicated that congener type was affected by light intensity and nutrient supply. In Planktothrix agardhii, the MC-LR and MC-RR ratio was affected by photon irradiance17 or amino acid availability (leucine and arginine)18. Monchamp et al.13 suggested that total nitrogen, water temperature, ammonium and dissolved organic nitrogen influenced the cyanobacterial community structure, which in turn resulted in differences in the dominant MC congener and the overall toxicity. Puddick et al.7 found the relative abundance of arginine-containing MC decreased as nitrate was depleted from the culture medium, indicating nitrogen played an important role in modulating the toxicity of Microcystis. Most of these studies examine the influence of environmental factors on the MC congeners in the lab and evidence for this influence in natural systems is limited. Knowledge on how environmental variables regulate MC congener abundance will assist in predicting the periods of greatest risk to human users exposed to these toxins7. It is important to understand what environmental parameters may regulate MC production but to determine what factors influence the variants of MC congeners produced by cyanobacterial blooms. Drowned-river mouth lakes are transitional zones between a lake and an inflowing river and have unique physical and hydrological dynamics19. West Michigan (USA) contains many drowned-river mouth lakes with histories of cyanobacteria blooms20,21. Studies of cyanotoxins in these lakes have been rare despite their high recreational use. Muskegon Lake and Bear Lake are both drowned river mouth systems, and are listed as a Great Lakes Area of Concern and require the restoration of Beneficial Use Impairments related to ‘Eutrophication or Undesirable Algae’ and ‘Restrictions on Drinking Water Consumption’ for delisting21. Detailed information concerning the nutrient chemistry, phytoplankton community dynamics, and cyanotoxins are necessary for delisting. Muskegon Lake, located on the eastern shore of Lake Michigan, has a long history of anthropogenic impairment22. Bear Lake is a hypereutrophic, shallow drowned river mouth system23 and the research into the cyanotoxins of cyanobacteria blooms in Muskegon Lake and Bear Lake began in the summer of 200624. The relative composition of microcystin differed between Bear and Muskegon Lakes despite their hydrologic connectivity. MC-LR and MC-RR percentage were equally abundant in Bear Lake, whereas MC-LR composed 54–87% of the total MC in Muskegon Lake24 suggesting the MC toxicity in the hypereutrophic Bear Lake is lower than mesotrophic/eutrophic Muskegon Lake due to the predominance of the more toxic MC-LR. Xie et al.24 hypothesized the greater percentage contribution of MC-LR than MC-RR was due to Muskegon Lake having a significantly lower summer temperature and different nitrogen chemistry than Bear Lake. However, the effect of temperature and other environmental factors on microcystin analog ratios in both lakes has not been evaluated in situ.
The aim of this study was to test, in natural ecosystems, the hypothesis that different N and P forms, alone or in combination with other environmental variables, influence the cyanobacterial community structure, the MC concentration, as well as the MC congener composition. Various forms of dialysis culture have been successfully used for studying a variety of phytoplankton species under laboratory as well as field conditions to investigate of species interactions and production of diffusible and non-diffusible products25,26. Such studies can allow the effects of environmental variables on microcystin production to be evaluated in the natural lake environment. We incubated dialysis bags containing Muskegon and Bear Lake’s phytoplankton in both lakes simultaneously to determine if differences in biotic and abiotic factors would influence MC production and congener ratios.
Results
Cyanobacterial assemblages
Phytoplankton communities were dominated by cyanobacteria. In total, 23 cyanobacterial taxa were identified in the dialysis bags. The plankton was dominated by the same cyanobacterial species for all experiments. The greatest biovolume of cyanobacteria was noted in the dialysis bags incubated in Muskegon Lake with Bear Lake water (MKBL) (Fig. 1). In July, the six dominant cyanobacteria identified in all bags were Aphanizomenon aphanizomenoides, Microcystis wesenbergii, Limnothrix redekei, Aphanocapsa pulchra, Lyngbya limnetica and Microcystis aeruginosa (Fig. 1). The A. aphanizomenoides biovolume in the experiments with Bear Lake water (triplicates for MKBL1-3; BLBL1-3) was significantly higher than with Muskegon Lake water (MKMK1-3, BLMK1-3) (p < 0.005). However, there were no differences found between all dialysis bags with Bear Lake water (p = 0.160) for this taxon. The M. wesenbergii biovolume with Bear Lake water was significantly higher than with Muskegon Lake water (p < 0.010). No differences were found between the individual experiments with Bear Lake water for this taxon (p = 0.020). L. redekei was present at greater biovolumes in the experiments with Bear Lake water (MKBL: 3.8 ± 2.3 × 109 μm3·mL−1; BLBL: 5.9 ± 2.9 × 109 μm3·mL−1) when compared to the dialysis bags with Muskegon Lake water (BLMK: 2.1 ± 2.0 × 108 μm3 mL−1; MKMK: 3.1 ± 0.9 × 108 μm3·mL−1). A. pulchra (BLBL: 6.6 ± 0.5 × 108 μm3·mL−1) and L. limnetica (MKBL: 1.7 ± 1.0 × 109 μm3·mL−1) were major contributors during July but were not present in August. M. aeruginosa was only observed in the bags with Muskegon Lake water (MKMK: 1.2 × 107 μm3·mL−11.2; BLMK: 3.2 × 108 μm3·mL−1) and in Bear Lake (9.1 × 108 μm3·mL−1). No cyanobacteria cells were found in the initial lake water of Muskegon Lake (MKI) and only a minor population of M. wesenbergii (1.1 × 107 μm3·mL−1) was found in the final lake water of Muskegon Lake (MKF).
Cyanobacteria population composition in dialysis bag experiments (mean ± SD).
(MKI: Muskegon Lake Initial; MKF: Muskegon Lake Final; MKMK: Muskegon Lake with Muskegon Lake water; MKBL: Muskegon Lake with Bear Lake water; BLI: Bear Lake Initial; BLF: Bear Lake Final; BLBL: Bear Lake with Bear Lake water; BLMK: Bear Lake with Muskegon Lake water. The taxonomic analyses were conducted with three replicates in July and five replicates in August.
In August (five replicates for the bags: MKBL1-5; BLBL1-5), the community structure shifted to Planktolyngbya limnetica and L. redekei, (Fig. 1) which became the dominant taxa (35.5% and 34.0%, respectively). Dominant species of the cyanobacterial communities throughout the two sampling periods in all the experiments were P. limnetica (maximum 1.7 × 1012 μm3·mL−1), L. redekei (maximum 1.5 × 1012 μm3·mL−1), M. wesenbergii (maximum 6.7 × 1011 μm3·mL−1), A. aphanizomenoides (maximum 4.9 × 1011 μm3·mL−1), and M. aeruginosa (maximum 3.6 × 1011 μm3·mL−1). Between July and August, significant differences were observed for mean biovolume of M. aeruginosa (p = 0.010) and L. redekei (p = 0.040). Mean biovolumes of A. aphanizomenoides (p = 0.140) and M. wesenbergii (p = 0.510) were not significantly different between dates. C. raciborskii trichomes were found in experiments with Bear Lake water and the ambient Bear Lake water, with the greatest biovolume of 1.6 × 1011 μm3·mL−1 observed.
Microcystin dynamics
In July, microcystins were detected in all samples analyzed in all dialysis bags but at lower concentrations in the experiments with Muskegon Lake phytoplankton (Fig. 2). The greatest total MC concentrations (20.1 ± 3.88 μg·L−1, range: 14.97–24.32 μg·L−1) were detected in bags incubated in Muskegon Lake with Bear Lake phytoplankton (MKBL1-3) (Fig. 2). MC-LR/MC-RR ratio of the bags initiated with Muskegon phytoplankton (MKMK; BLMK) and the ambient Muskegon Lake phytoplankton (MKI 7/16, MKF 7/22) were significantly higher than the other dialysis bags (BLBL; MKBL) and ambient Bear Lake water (BLI 7/16; BLF 7/22) (p = 0.010). Percent contributions of the MC-RR, MC-LR, and MC-YR congeners to total MC concentrations in the bags with Bear Lake phytoplankton (MKBL1-3, BLBL1-3) ranged from 53.8–60.6%, 32.4–38.4%, and 6.6–8.1%, respectively. Percent contributions of the MC-RR, MC-LR, and MC-YR congeners to total MC concentrations in the bags initiated with Muskegon Lake phytoplankton (MKMK1-3, BLMK1-3) ranged from 18.9–31.2%, 57.0–71.1%, and 8.2–11.9%, respectively. No statistically significant difference in MC-LR/MC-RR ratio was observed between the bags with Bear Lake phytoplankton and the ambient phytoplankton of Bear Lake (p = 0.100), but significant differences in the bags with Muskegon Lake phytoplankton and the ambient phytoplankton of Muskegon Lake (p = 0.020) were observed.
Microcystin analogue concentrations and MC-LR/MC-RR ratio in dialysis bags.
(MKI; Muskegon Lake Initial, MKF; Muskegon Lake Final, MKMK; Muskegon Lake with Muskegon Lake water, MKBL; Muskegon Lake with Bear Lake water, BLI; Bear Lake Initial, BLF; Bear Lake Final, BLBL; Bear Lake with Bear Lake water, and BLMK; Bear Lake with Muskegon Lake water.)
In August, the mean concentrations of total MC (7.04 ± 0.73 μg·L−1, range: 5.77–7.97 μg·L−1) in bags in Muskegon Lake initiated with Bear Lake phytoplankton (MKBL1-3) were also significantly higher than the MC in other bags (p < 0.001) (Fig. 2). MC-LR/MC-RR in the bags initiated with Muskegon phytoplankton (MKMK; BLMK) and the ambient phytoplankton from Muskegon Lake (MKI 8/16, MKF 8/22) were significantly higher than the other bags (BLBL; MKBL) and Bear Lake (BLI 8/16; BLF 8/22) (p < 0.001). Percent contributions of the MC-RR, MC-LR, and MC-YR congeners to the total MC concentrations in bags initiated with Bear Lake phytoplankton (MKBL1-3, BLBL1-3) ranged from 52.7–66.0%, 29.3–41.2%, and 3.82–8.61%, respectively. Percent contributions of the MC-RR, MC-LR, and MC-YR congeners to total MC concentrations in the bags initiated with Muskegon Lake phytoplankton (MKMK1-3, BLMK1-3) ranged from 28.2–36.0%, 54.4–66.2%, and 5.06–10.7%, respectively.
In both months, MC concentrations correlated with the biomass of A. aphanizomenoides (R2 = 0.312, p < 0.001, Spearman’s), M. wesenbergii (R2 = 0.121, p = 0.038), L. limnetica (R2 = 0.131, p = 0.030), but not correlated with L. redekei (R2 = 0.072, p = 0.115), A. pulchra (R2 = 0.070, p = 0.121), P. limnetica (R2 = 0.000, p = 0.984) and M. aruginosa (R2 = 0.004, p = 0.735), C. raciborskii (R2 = 0.041, p = 0.239) (Table 1). No MC-LA and CYN were detected throughout all the experiment.
Environmental factors
Physicochemical parameters showed little temporal and spatial variation in Bear Lake and Muskegon Lake (Table 2). In both months, the SRP concentration was below the detection limit during the sampling period. The concentrations of nitrate (NO3-N) and ammonia (NH3-N) were higher in Muskegon Lake and the corresponding bags (MKMK; MKBL) than in Bear Lake and the corresponding bags (BLBL; BLMK) (p < 0.010 and p < 0.030, respectively). The MC concentrations were not correlated with the nitrate concentration (R2 = −0.422, p = 0.509) or ammonia concentration (R2 = −0.616, p = 0.150). The concentrations of TP and TN were higher in the Bear Lake and the bags initiated with Bear Lake phytoplankton (BLI; BLF; MKBL; BLBL) than Muskegon and the bags initiated with Muskegon Lake phytoplankton (MKI; MKF; MKMK; BLMK) (p < 0.010). The MC concentrations were correlated with the TN (R2 = 0.889, p < 0.001) and TP (R2 = 0.768, p = 0.020). There were no statistically significant differences noted between bags initiated with Muskegon Lake phytoplankton (MKMK; BLMK) and Bear Lake phytoplankton (BLBL; MKBL) during both months for Cl− (p > 0.130), SO42− (p > 0.050), Hardness (p > 0.230), alkalinity (p > 0.310). MC concentrations were not correlated with Cl− (R = −0.200, p = 0.880), SO42− (R = −0.224, p = 0.860), hardness (R2 = −0.200, p = 0.880) and alkalinity (R2 = 0.173, p = 0.910).3
We measured the environmental factors in Bear Lake and Muskegon Lake in August (Fig. 3). Light intensity ranged from 181.5–1147.0 μmol·m−2·s−1 in Muskegon Lake and 107.5–526.8 μmol·m−2·s−1 in Bear Lake (Fig. 3). Statistically significant differences were noted between Bear Lake and Muskegon Lake for light intensity (p < 0.001), temperature (p < 0.001) and turbidity (p < 0.001), but not for TDS (p = 0.280) (Fig. 3).
Discussion
Several research groups have studied how environmental parameters affect the dominance of cyanobacteria and total MC concentrations in lakes11,13,27. Also, some studies described the relationship of bloom community dynamics and the MC congener concentration and composition6,13,28. About MC congers, Tonk et al.17 suggested that the ratio of MC variants changed in response to differing light intensities; de Figueiredo et al.29 found out that higher temperatures enhanced MC-RR production, whereas lower temperatures favored MC-LR synthesis. While Monchamp et al.13 suggested that environmental factors did not appear to affect MC congener composition directly but there were significant associations between specific MC congeners and particular species. In our experiment, there was a significant difference in total MC concentrations between all the treatments. Total MC concentrations in the bags incubated in Muskegon Lake with Bear Lake water (MKBL) were significantly higher than the other treatments. No significant differences between the cyanobacteria biovolume in all the bags with Bear Lake water were observed and MC-LR/MC-RR ratios from the treatments with the corresponding lakes were similar during the study period.
According to previous studies30,31,32, MC production was correlated with algal species and cell growth. M. aeruginosa has been classified as a major MC producer in previous research33,34. In July, the greatest total MC concentrations (20.1 ± 3.88 μg·L−1, range: 14.97–24.32 μg·L−1) were found in bags without M. aeruginosa present. In addition, MC concentrations were not correlated with M. aruginosa biomass in both months, indicating that there were other cyanobacteria strains producing MC. MC concentrations were found to be correlated with M. wesenbergii in the current experiment. In term of MC production by M. wesenbergii, previous studies yielded contradictory conclusions. Henriksen35 found that M. wesenbergii was dominated in hepatotoxic Microcystis blooms of Danish lakes. While Watanabe36 concluded that M. wesenbergii has generally been considered as nontoxic. By both molecular and chemical methods, recent studies showed that M. wesenbergii lacked MC production genes in Germany and other European lakes37,38 and in China39. Also, in our early MC investigation in seven lakes of Michigan, the MC concentrations were not correlated with the biomass M. wesenbergii (unpublished data). Based on the literature findings in spite of the observed correlation, it was likely that M. wesenbergii was a nontoxic species in our experiments.
The traditional genus Aphanizomenon comprises a group of filamentous nitrogen-fixing cyanobacteria of which several members are able to develop blooms and to produce toxic metabolites (cyanotoxins), including hepatotoxins (microcystins), neurotoxins (anatoxins and saxitoxins) and cytotoxins (cylindrospermopsin)40. The species of Sphaerospermopsis aphanizomenoides isolated from Lake Oued Mellah was reported to contain MCs, namely four compounds displaying a retention time similar to that of MC-LA, LY, LW or LF in HPLC-PDA chromatograms41. In this study, MC concentrations correlated with the biomass of A. aphanizomenoides in both months indicating that A. aphanizomenoides is a potential MC producer. A. aphanizomenoides was considered to be salinity-tolerant42, requires high water temperature43, and the biomass of A. aphanizomenoides was found to be significantly related to the water temperatures44. This cyanobacterium has been detected in water bodies in several countries44 and has been expanding its range into more half regions of European45,46. A. aphanizomenoides has not been linked to MC production with the exception of a study also conducted in Bear Lake where the organism was listed as the dominant cyanobacteria species and a suspected MC producer47. In consideration of the strong statistical correlation between A. aphanizomenoides biovolume and MC production occurring in the same lake, our study assumes that A. aphanizomenoides may be a MC producer. Genetic studies still need to be performed to determine if toxin producing genes are present in this organism.
MC production also was influenced by environmental parameters32. Some studies suggested that the environmental parameters, i.e., phosphorus, nitrogen, temperature, light etc., affect the MC production and the growth of M. aeruginosa in continuous cultures, laboratory batch, or in the field11,12,48. Environmental parameters may affect MC concentrationsin two principal ways: regulating MC production by the toxigenic strains or regulating the population of MC-producing strains49. Sivonen10 indicated that MC production by Oscillatoria agardhii correlated with high nitrate concentration (0.42–0.84 mg·N/L) and low light intensity (12–95 μmol·m−2·s−1). While Jiang et al.34 suggested that light and iron had significant interactive effect on MC production. For Microcystis PCC 7806, Wiedner et al.49 indicated that the maximum MC concentrations were reached at light intensities of 40 μmol·m−2·s−1 but a decline in MC production and cellular MC content were observed by further increasing the irradiance during lab experiments. In addition, for M. aeruginosa W334, Hesse and Kohl16 found that celluar MC-LR concentrations decreased at a growth rate at 80 μmol·m−2·s−1, but for M. aeruginosa W368, MC-LR and MC-YR, cellular contents increased at 100 μmol·m−2·s−1. Yang et al.50 found out that MC production decreased significantly when the strain was exposed to UV-B radiation. For P. agardhii, Sivonen10 noted that higher MC concentrations were produced at lower irradiances (12 and 24 μmol·m−2·s−1) rather than at higher numbers (50 and 95 μmol·m−2·s−1). Monchamp et al.13 indicated that water temperature, TN, ammonium and DON can influence the cyanobacterial population structure, which resulted in the differences of the dominant MC congeners and the toxicity. It seemed that the diverse effects of light on the MC production depend on the cyanobacterial species and on the MC analogue. Currently, although opinions vary, MC production appears to be linked to N availability27,51,52 and functions to alleviate oxidative stress during high light conditions53,54.
In this study, MC-LR/MC-RR ratios varied with sample origin but not with lake of incubation, indicating that water temperature, light and turbidity were not the reasons for the difference of the MC-LR/MC-RR ratio. Van de Waal et al.27 studied how nitrogen pulse affect the MC variants of P. agardhii and found out MC-RR increased strongly, while MC-LR increased weakly after the nitrogen pulse. They speculated Microcystis and other MC-producing algae would respond similarly. In this study, we observed that the biovolume of A. aphanizomenoides followed the increase of MC production. A. aphanizomenoides is able to fix molecular nitrogen (diazotrophy) and in this study, we found low levels of NO3-N and NH4-N along with high levels of TN (Table 1). These numbers are typical for an environment in which N2 fixation takes place. Hence, it is possible that with fixed N2 made available for MC producing strains, both the overall MC content and the MC-LR/MC-RR ratio should be expected to change. With A. aphanizomenoides present, the limiting nutrient is supplied by N2 fixation may have resulted in the relative increase of MC-RR and MC-LR (Fig. 2). In Muskegon Lake water, the nutrient balance may not be suitable for N-fixation due to higher NO3-N concentrations since nitrate can suppress nitrogenase in some cyanobacterium55. Hence, the higher NO3-N concentrations were a possible factor for the increase of MC concentrations in the dialysis bags. Also, light was considered an important factor affecting MC production as light intensity can regulate the transcription of the MC-synthesizing gene56. In the present study, Muskegon Lake had lower temperature, higher light intensity, and lower turbidity than Bear Lake. Since the growth of A. aphanizomenoides requires higher water temperatures, the lower thermal profile observed in Muskegon Lake might not be conducive for the increase toxin production. In this study, the light intensity of Bear Lake (average: 397.2 μmol·m−2·s−1) was significantly lower than Muskegon Lake (800.1 μmol·m−2·s−1). Low-light conditions were generated by two main factors: water depth and turbidity57. Since we incubated all the dialysis bags in the same depth (1 m) of the two lakes, the higher turbidity of Bear Lake appears to be responsible for the lower light intensity. The high light intensity of Muskegon Lake appears be another reason for the increase of MC concentrations in the dialysis bags with Bear Lake water incubated in Muskegon Lake.
Oh et al.58 suggested that MC-LR/MC-RR ratio can increase with severe P-limited conditions. Sas et al.59 indicated that phytoplankton growth was P-limited if FRP was <10 μg·L−1 of the growing season. In this study, SRP of the two lakes and all the dialysis bags were less than 5 μg·L−1, TP in Muskegon Lake and the bags with Muskegon Lake water were all less than 50 μg·L−1, while TP in Bear Lake and bags with Bear Lake water were ~100 μg·L−1. The difference in bioavailable TP concentrations may be one reason for the dissimilarity of the MC-LR to MC-RR ratio of Muskegon Lake and Bear Lake. Furthermore, other factors which were not specifically investigated during the present study (e.g. turbulence, zooplankton predation) could also have an influence on the abundance of different microcystin congeners and we will do the further research in this field.
Methods
Experimental design
Experiments were conducted with water collected from Bear Lake and Muskegon Lake. Bear Lake has a surface area of 1.66 km2, an average depth of 2.14 m, and a maximum depth of 3.66 m23. Bear Lake discharges to Muskegon Lake through a narrow navigation channel at a rate of 0.9 m3/s and has a mean hydraulic residence time of 30 days60. Muskegon Lake is a mesotrophic/eutrophic, drowned river mouth system with a surface area of 16.6 km2 and an average depth of 7.1 m, with a maximum depth of 23 m61. Muskegon Lake discharges to Lake Michigan at a rate of 55.5 m3/s and has a mean hydraulic residence time of 25 days61. The Muskegon River accounts for 95% of the tributary inputs to Muskegon Lake62. Both lakes are well mixed24.
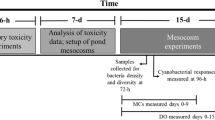
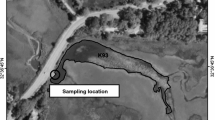
Dialysis bags were filled with lake water and phytoplankton from five meters away from Bear Lake Dock and 5 meters away from Muskegon Lake Barge (Fig. 4) at 1 meter depth in July 19th 2010. All measurements occurred between 9:00 and 11:00 AM. The bags were constructed of Spectra/Por 5 dialysis tubing (12–14 K MWCO, 140 mm flat width; Spectrum Laboratories, CA) and contained approximately 500 ml of lake water and were completely sealed. Triplicate dialysis bags of water from each lake were attached to a support cage and incubated for 7 days in Bear Lake and Muskegon Lake at 1 m depth (the maximum depth of Bear Lake shore is 1.5 m). Dialysis bag samples were identified as MKMK (Muskegon Lake with Muskegon Lake water), MKBL (Muskegon Lake with Bear Lake water), BLBL (Bear Lake with Bear Lake water), and BLMK (Bear Lake with Muskegon Lake water). On August 16th, the samples were taken and incubated in the same location. To confirm the data of July was not random, we use 5 replicates of water from each lake at this time.
Sampling locations in Bear Lake Dock and Muskegon Lake Barge.
This map was generated in ESRI ArcMap 10 (Environmental Systems Resource Institute, ArcMap 10 ESRI, Redlands, California, USA, http://www.esri.com/).
For chemical and biological analysis, water samples were collected near the support cages at the beginning (MKI and BLI, respectively) and end of the experiments (MKF and BLF, respectively). In addition, daily in situ measurements of Photosynthetically Active Radiation (PAR) were measured with a LiCor Li-193SA (spherical quantum sensor) and temperature, turbidity, and total dissolved solids (TDS) were measured with a YSI 6600. All in situ measurements were conducted adjacent to the dialysis bags at 1 m depth.
After the 7-day incubation period, the bags were mixed well prior to sampling and a 25 ml aliquot from each dialysis bag was withdrawn for phytoplankton analysis. The remaining water was stored immediately in a portable refrigerator (around 4 °C) and composited into a single sample for nutrient analysis.
Chemical analysis
Three 100 ml aliquots from each dialysis bag were immediately placed on ice and returned to the lab for filtration on a 0.7 μm Whatman GF/F glass microfiber filter (Fisher Scientific cat # 09-874-64) and stored at −20 °C for cyanotoxin analysis. According to Fastner et al.63 and Dyble et al.64, toxin samples were lyophilized first and then sonicated in 75% aqueous methanol. MC analogues (MC-LR, MC-RR, MC-YR, MC-LA; Sigma-Aldrich) and cylindrospermopsin (CYN) (Sigma-Aldrich) analysis was performed by High-Performance Liquid Chromatography coupled Mass Spectrometry (HPLC/MS) using a Thermo Surveyor MSQ Single Quadrupole Mass Selective Detector and Thermo Spectrasystem gradient chromatographic system according to a method described by Barco et al.65. Total MC concentrations were reported as the sum of all congeners (HPLC/MS-Total).
Total Kjeldahl nitrogen (TKN-N) and ammonia (NH3-N) were analyzed on a BRAN+LUEBBE Autoanalyzer66. Nitrate (NO3-N), total phosphorus (TP-P), and soluble reactive phosphorus (SRP-P) were analyzed on an ion chromatograph (detections limit: 0.005 mg/L, Standard Methods 4100 C)67.
Phytoplankton identification
Phytoplankton samples were preserved with 1% acidic Lugol’s solution. Algae were identified and enumerated utilizing a Nikon Eclipse TE200 inverted microscope68. At least 200–300 algal units (cells or filaments) were counted in all the samples. The cell volume of each species was calculated by applying the appropriate geometric formulae69. The detailing for cell density calculations please see Table 3.
Statistical calculation
Statistical analyses were conducted with SPSS version 12.0.1 (SPSS, Inc. Chicago IL, USA). The non-parametric Wilcoxon sign test was used to evaluate MC concentrations differences between the bags and ambient samples in July and August as data were not normally distributed. Differences in cyanobacterial biovolume and MC concenration between the bags and ambient samples were examined with the non-parametric Wilcoxon sign test (a = 0.05). Statistical similarity was evaluated with the Mann Whitney U test (a = 0.05) and multiple correlations were performed with Spearman’s Rank-Order Correlation (a = 0.05). To test if the two months (July and August) had significantly different cyanobacterial assemblages, samples were analyzed with the nonparametric-analysis of similarity (ANOSIM, Clarke70). This method tests for significant differences (a = 0.05) between two or more groups using the rank order of the samples similarity matrix based on the Bray-Curtis similarity coefficient. To examine the differences between MC-LR/MC-RR ratio, the Mann Whitney U test was used (differences being significant at p < 0.05). To examine the differences between environmental factors, the Mann Whitney U test was used (differences being significant at p < 0.05).
Conclusion
Our data suggest that differences in total phosphorus concentrations were a reason for the dissimilarity of the MC-LR/MC-RR ratio between Muskegon Lake and Bear Lake. The higher light intensity due to lower turbidity and NO3-N concentrations in Muskegon Lake were two factors contributing to an increase of total MC production.
Additional Information
How to cite this article: Xie, L. et al. The impact of environmental parameters on microcystin production in dialysis bag experiments. Sci. Rep. 6, 38722; doi: 10.1038/srep38722 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Paerl, H. W., Rd, F. R., Moisander, P. H. & Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci World J 1, 76–113 (2001).
Chen, J., Xie, P., Zhang, D., Ke, Z. & Yang, H. In situ studies on the bioaccumulation of microcystins in the phytoplanktivorous silver carp (Hypophthalmichthys molitrix) stocked in Lake Taihu with dense toxic Microcystis blooms. Aquaculture 261, 1026–1038 (2006).
Xie, L., Yokoyama, A., Nakamura, K. & Park, H. Accumulation of microcystins in various organs of the freshwater snail Sinotaia histrica and three fishes in a temperate lake, the eutrophic Lake Suwa, Japan. Toxicon 49, 646–652 (2007).
Zhang, D., Xie, P., Liu, Y. & Qiu, T. Transfer, distribution and bioaccumulation of microcystins in the aquatic food web in Lake Taihu, China, with potential risks to human health. Sci Total Environ 407, 2191–2199 (2009).
Stirling, D. J. & Miles, C. O. Marine algal toxin and cyanobacterial toxin mass lists. http://www.toxinology.no/Downloads.aspx. Accessed 11 June 2015 (2015).
Cerasino, L. & Salmaso, N. Diversity and distribution of cyanobacterial toxins in the Italian subalpine lacustrine district. Oceanol Hydrobiol St 41, 54–63 (2012).
Puddick, J., Prinsep, M. R., Wood, S. A., Cary, S. C. & Hamilton, D. P. Modulation of microcystin congener abundance following nitrogen depletion of a Microcystis batch culture. Aquat Ecol 50, 235–246 (2016).
Carmichael, W. W. Toxins in freshwater algae in Handbook of natural toxins Vol. III: Marine toxins and venoms (ed Tu, A. T. & Decker, Marcel ) 121–147 (1988).
Sivonen, K. & Jones, G. Cyanobacteria toxins in Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management (eds Chorus, Ingrid & Bartram, Jamie ) 44–111 (London: Spon, 1999).
Sivonen, K. Effects of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production by Oscillatoria agardhii strains. Appl Environ Microb 56, 2658–2666 (1990).
Wang, Q. et al. Factors affecting temporal and spatial variations of microcystins in Gonghu Bay of Lake Taihu, with potential risk of microcystin contamination to human health. Sci World J 10, 1795–1809 (2010).
Yu, L. et al. The dynamics of Microcystis genotypes and microcystin production and associations with environmental factors during blooms in Lake Chaohu, China. Toxins 6, 3238–3257 (2014).
Monchamp, M.-E., Pick, F. R., Beisner, B. E. & Maranger, R. Nitrogen forms influence microcystin concentration and composition via changes in cyanobacterial community structure. Plos One 9, e85573 (2014).
Gleason, F. & Wood, J. Secondary metabolism in the cyanobacteria in The cyanobacteria (ed Fay, P. & Van Baalen, C. ) 437–452 (Elsevier, Amsterdam, the Netherlands, 1987).
Rapala, J., Sivonen, K., Lyra, C. & Niemelä, S. I. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl Environ Microb 63, 2206–2212 (1997).
Hesse, K. & Kohl, J.-H. Effects of light and nutrient supply on growth and microcystin concentration of different strains of Microcystis aeruginosa in Cyanotoxins: occurrence, causes, consequences (ed. Chorus, I. ) 152–158 (Springer: Heidelberg/Berlin, Germany, 2001).
Tonk, L. et al. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl Environ Microb 71, 5177–5181 (2005).
Tonk, L. et al. Amino acid availability determines the ratio of microcystin variants in the cyanobacterium Planktothrix agardhii. FEMS Microb Ecol 65, 383–390 (2008).
Gillett, N. D., Luttenton, M. R. & Steinman, A. D. Spatial and temporal dynamics of phytoplankton communities in a Great Lakes drowned river-mouth lake (Mona Lake, USA). J Limnol 74, 453–466 (2015).
Hong, Y., Steinman, A., Biddanda, B., Rediske, R. & Fahnenstiel, G. Occurrence of the toxin-producing cyanobacterium Cylindrospermopsis raciborskii in Mona and Muskegon Lakes, Michigan. J Great Lakes Res 32, 645–652 (2006).
Steinman, A. D. et al. Current status and trends in Muskegon Lake, Michigan. J Great Lakes Res 34, 169–188 (2008).
Nelson, W. A. & Steinman, A. D. Changes in the benthic communities of Muskegon Lake, a Great Lakes Area of Concern. J Great Lakes Res 39, 7–18 (2013).
Wilson, A. E. et al. Genetic variation of the bloom-forming Cyanobacterium Microcystis aeruginosa within and among lakes: implications for harmful algal blooms. Appl Environ Microb 71, 6126–6133 (2005).
Xie, L. et al. The influence of environmental conditions and hydrologic connectivity on cyanobacteria assemblages in two drowned river mouth lakes. J Great Lakes Res 37, 470–479 (2011).
Furnas, M. J. An evaluation of two diffusion culture techniques for estimating phytoplankton growth rates in situ. Mar Biol 70, 63–72 (1982).
Jezbera, J., Horňák, K. & Šimek, K. Prey selectivity of bacterivorous protists in different size fractions of reservoir water amended with nutrients. Environ Microbiol 8, 1330–1339 (2006).
Van de Waal, D. B. et al. The ecological stoichiometry of toxins produced by harmful cyanobacteria: an experimental test of the carbon-nutrient balance hypothesis. Eco Lett 12, 1326–1335 (2009).
Miller, M. A. et al. Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from land to Sea Otters. Plos One 5, e12576 (2010).
de Figueiredo, D. R., Azeiteiro, U. M., Esteves, S. M., Gonçalves, F. J. M. & Pereira, M. J. Microcystin-producing blooms—a serious global public health issue. Ecotox Environ Safe 59, 151–163 (2004).
Long, B. M., Jones, G. J. & Orr, P. T. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl Environ Microb 67, 278–283 (2001).
Rolland, A., Bird, D. F. & Giani, A. Seasonal changes in composition of the cyanobacterial community and the occurrence of hepatotoxic blooms in the eastern townships, Québec, Canada. J Plankton Res 27, 683–694 (2005).
Wicks, R. J. & Thiel, P. G. Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African reservoir. Environ Sci Technol 24, 1413–1418 (1990).
Botes, D. P. et al. The structure of cyanoginosin-LA, a cyclic heptapeptide toxin from the cyanobacterium Microcystis aeruginosa. J Chem Soc, Perkin Trans 1, 2311–2318 (1984).
Jiang, Y., Ji, B., Wong, R. & Wong, M. Statistical study on the effects of environmental factors on the growth and microcystins production of bloom-forming cyanobacterium—Microcystis aeruginosa. Harmful Algae 7, 127–136 (2008).
Henriksen, P. Microcystin profilesand contents in Danish populations of cyanobacteria/blue-green algae as determined by HPLC. Phycologia 35, 102–110 (1996).
Watanabe, M. Isolation, cultivation and classification of bloom-forming Microcystis in Japan in Toxic Microcystis Vol. 2 (eds Watanabe, M. F., Harada, K., Carmichael, W. W. & Fujiki, H. P. ) 13–34 (CRC Press, Boca Raton, 1996).
Kurmayer, R., Dittmann, E., Fastner, J. & Chorus, I. Diversity of microcystin genes within a population of the toxic cyanobacterium Microcystis spp. in lake Wannsee (Berlin, Germany). Microb Ecol 43, 107–118 (2002).
Via-Ordorika, L. et al. Distribution of microcystin-producing and non-microcystin-producing Microcystis sp. in European freshwater bodies: detection of microcystins and microcystin genes in individual colonies. Syst Appl Microbiol 27, 592–602 (2004).
Xu, Y. et al. Non-microcystin producing Microcystis wesenbergii (Komárek) Komárek (Cyanobacteria) representing a main waterbloom-forming species in Chinese waters. Environ Pollut 156, 162–167 (2008).
Cirés, S. & Ballot, A. A review of the phylogeny, ecology and toxin production of bloom-forming Aphanizomenon spp. and related species within the Nostocales (cyanobacteria). Harmful Algae 54, 21–43 (2016).
Sabour, B. et al. Contributed Article Dynamics and toxicity of Anabaena aphanizomenoides (Cyanobacteria) waterblooms in the shallow brackish Oued Mellah lake (Morocco). Aquat Ecosyst Health 8, 95–104 (2005).
Apte, S., Fernandes, T., Iyer, V. & Alahari, A. Molecular basis of tolerance to salinity and drought stresses in photosynthetic nitrogen-fixing cyanobacteria in Plant molecular biology and biotechnology (eds K., K. & Singhal Tewari, G. S. ) 258–268 (Narosa Publications, New Delhi, 1997).
Kovacs, A., Koncz, E. & Voros, L. Light dependent species succession of heterocystous cyanobacteria. Phycologia 36, 53–53 (1997).
Stefaniak, K. & Kokocinski, M. Occurrence of invasive cyanobacteria species in polimictic lakes of the Wielkopolska Region [Western Poland]. Oceanol Hydrobiol St 34, 137–148 (2005).
Stüken, A. et al. Distribution of three alien cyanobacterial species (Nostocales) in northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides. Phycologia 45, 696–703 (2006).
Maileht, K. et al. Water colour, phosphorus and alkalinity are the major determinants of the dominant phytoplankton species in European lakes. Hydrobiologia 704, 115–126 (2013).
Backer, L. C. et al. Recreational exposure to low concentrations of microcystins during an algal bloom in a small lake. Mar Drugs 6, 389–406 (2008).
Jacoby, J. M., Collier, D. C., Welch, E. B., Hardy, F. J. & Crayton, M. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can J Fish Aquat Sci 57, 231–240 (2000).
Wiedner, C. et al. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl Environ Microb 69, 1475–1481 (2003).
Yang, Z., Kong, F., Shi, X., Yu, Y. & Zhang, M. Effects of UV-B radiation on microcystin production of a toxic strain of Microcystis aeruginosa and its competitiveness against a non-toxic strain. J Hazard Mate 283, 447–453 (2015).
Horst, G. P. et al. Nitrogen availability increases the toxin quota of a harmful cyanobacterium, Microcystis aeruginosa. Water Res 54, 188–198 (2014).
Harke, M. J. et al. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54, 4–20 (2016).
Zilliges, Y. et al. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. Plos One 6, e17615 (2010).
Paerl, H. W. & Otten, T. G. Harmful cyanobacterial blooms: causes, consequences, and controls. Microb Ecol 65, 995–1010 (2013).
Ramos, J. L. & Guerrero, M. G. Involvement of ammonium metabolism in the nitrate inhibition of nitrogen fixation in Anabaena sp. strain ATCC 33047. Arch Microbiol 136, 81–83 (1983).
Kim, H. R., Kim, C. K., Ahn, T. S., Yoo, S. & Lee, D. H. Effects of temperature and light on microcystin synthetase gene transcription in Microcystis aeruginosa. Key Eng Mater 277–279, 606–611 (2005).
Sedmak, B. & Kosi, G. The role of microcystins in heavy cyanobacterial bloom formation. J Plankton Res 20, 691–708 (1998).
Oh, H.-M., Lee, S. J., Jang, M.-H. & Yoon, B.-D. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat. Appl Environ Microb 66, 176–179 (2000).
Sas, H. Lake restoration by reduction of nutrient loading: expectation, experiences, extrapolations 497 (St. Augustin: Academia Verlag Richarz GmbH, 1989).
MDNRE. Total maximum daily load for phosphorus for Bear Lake Muskegon County (eds Michigan Department of Natural Resources and the Environment) (2008).
Freedman, P., Canale, R. & Auer, M. The impact of wastewater diversion spray irrigation on water quality in Muskegon County lakes. Vol. EPA 905/9–79–006-A (eds US Environmental Protection Agency) (Washington, D. C, 1979).
Carter, G. S., Nalepa, T. F. & Rediske, R. R. Status and trends of benthic populations in a coastal drowned river mouth lake of Lake Michigan. J Great Lakes Res 32, 578–595 (2006).
Fastner, J., Flieger, I. & Neumann, U. Optimised extraction of microcystins from field samples—a comparison of different solvents and procedures. Water Res 32, 3177–3181 (1998).
Dyble, J., Fahnenstiel, G. L., Litaker, R. W., Millie, D. F. & Tester, P. A. Microcystin concentrations and genetic diversity of Microcystis in the lower Great Lakes. Environ Toxicol 23, 507–516 (2008).
Barco, M., Rivera, J. & Caixach, J. Analysis of cyanobacterial hepatotoxins in water samples by microbore reversed-phase liquid chromatography–electrospray ionisation mass spectrometry. J Chromatogr A 959, 103–111 (2002).
Kopp, J. F. & McKee, G. D. Methods for chemical analysis of water and wastes USEPA Report No. EPA-600/4-79020 (US Environ. Prot. Agenoy, Washington, DC, 1983).
Clesceri, L. S., Greenberg, A. E. & Eaton., A. D. Standard methods for the examination of water and wastewater (American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC, 1998).
Agency, U. S. E. P. Standard operating procedure for phytoplankton analysis in Lake Michigan mass balance method 087 (Great Lakes national program office, United States Environmental Protection Agency, 1997).
Hillebrand, H., Dürselen, C. D., Kirschtel, D., Pollingher, U. & Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J Phycol 35, 403–424 (1999).
Clarke, K. R. Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18, 117–143 (1993).
Acknowledgements
Funding for this work was supported by the postdoctoral scholarship of Annis Water Resources Institute. Field and laboratory support was provided by Elizabeth Tromp and Andrew Sisson.
Author information
Authors and Affiliations
Contributions
L.Q.X. wrote the main manuscript text. L.Q.X., R.R.R., N.D.G., J.P.O. and B.S. designed and conducted the experiments and collected and analyzed the data. L.Q.X., Q.J.X. and R.R.R. checked and modified the manuscript text. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xie, L., Rediske, R., Gillett, N. et al. The impact of environmental parameters on microcystin production in dialysis bag experiments. Sci Rep 6, 38722 (2016). https://doi.org/10.1038/srep38722
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38722
- Springer Nature Limited
This article is cited by
-
Occurrence and diversity of cyanotoxins in Greek lakes
Scientific Reports (2018)