Abstract
Silver nanoclusters were synthesized and passivated by glutathione (GSH) ligand, with high aqueous stability and powerful red fluorescence and UV-vis yellow colour. Importantly, the specific recognition of the AgNCs was modulated from Hg2+ ions to Cu2+ ions upon the GSH passivation, of which the unique GSH-Cu2+ chelating reaction could conduct the fluorescence quenching of AgNCs. Strong UV-vis absorbance of GSH-passivated AgNCs could also be realized depending on the Cu2+ levels. Moreover, the Cu2+-induced loss of fluorescence and UV-vis absorbance of GSH-passivated AgNCs could be well restored by using stronger Cu2+ chelating agent. A simultaneous and reversible fluorimetric and colorimetric sensing method was thereby developed for probing Cu2+ ions in blood with high sensitivity and selectivity. Subsequently, the fluorescence-trackable imaging for live tissues and cells was demonstrated towards the analysis Cu2+ ions using GSH-passivated AgNCs as the fluorescent probes. This study indicates that the use of functional ligands like GSH could not only modulate the specific ion recognition of AgNCs, but also endow them the high aqueous stability and powerful red fluorescence towards the wide applications for ion sensing and biological imaging in the complicated media like blood.
Similar content being viewed by others
Introduction
In recent years, metal nanoclusters (NCs) with some distinct optical and catalytic properties have obtained increasing applications in the fields of chemical sensing, molecular labelling, biological imaging and catalysis1,2,3,4. In particular, a variety of noble metal NCs, most known as AuNCs, AgNCs and their alloy NCs, have been applied for detecting some toxic metal ions of great importance5,6,7,8,9. Moreover, many synthesis methodologies have been developed for preparing these unique fluorescent materials by using different organic or biological templates such as proteins, peptides, polyelectrolyte and DNAs5,10,11. For example, luminescent AuNCs and Au@AgNCs were prepared in the protein matrix by the sonochemistry route for probing copper ions in water5. Polyelectrolyte was employed as the template to synthesize highly fluorescent AgNCs for the detection of Hg2+ and Cu2+ ions10. DNAs were also reported as the stabilizer for fabricating AgNCs to probe Cu2+ ions11. In these studies, these templates have only been recognized as the stabilizers in the synthesis of the fluorescent species, with the limited functional diversifications like the specific ion recognition. Particularly, how they could play the role in modulating the specific recognitions or responses of noble metal NCs to the meaningful metal ions have hardly been explored systematically. Therefore, the synthesis of metal nanoclusters with the specific recognition modulated by ligand passivation is an attractive but challenging target to pursue.
As the hazardous heavy metal ions in environment, copper ions may bring deleterious effects with too high concentrations in tissues. For example, the long-term exposure to copper ions of high levels can lead to cellular toxicity and liver or kidneys damage12,13. So far, many modern detection methods have been applied for targeting copper ions such as electrochemical detection, fluorescence analysis and colorimetric assay5,14,15. For example, AgNCs have been widely documented for the fluorescent analysis of Cu2+ ions and/or Hg2+ ions, which might, however, be trapped by the interferences from co-existing metal ions that may challenge the specific ion detections16,17. Also, most of these methods might encounter with the low detection sensitivity and poor probing abilities against the background interferences. Therefore, it is of great interest to develop a simple, rapid and highly sensitive detection method to explore copper ions in some complicated media especially in human body fluids (i.e., blood).
Glutathione (GSH), a ubiquitous antioxidant in human and plant cells, is a tri-peptide consisting of glutamic acid, cysteine and glycine units. It possesses the reactive thiol groups with good affinity to metal ions and enjoys amine and carboxylate groups for coupling with other molecules or ions of great interest18. As a result, GSH has been applied as an effective stabilizer in the synthesis of some metal NCs like AgNCs19 and AuNCs20. Also, GSH was demonstrated to possess the high affinity or chelating ability to Cu2+ ions21. In the present work, GSH was chosen alternatively as an example of passivation ligands to work with dihydrolipoic acid (DHLA) to synthesize water-soluble GSH-passivated AgNCs with considerably strong red fluorescence and yellow colour. Importantly, the specific ion recognition of AgNCs could be thus modulated to Cu2+ ions upon the GSH passivation, in contrast to the general AgNCs with response to Hg2+ ions7. Comparing to most of the detection methods documented for Cu2+ ions, the so developed detection method could achieve the selective analysis for Cu2+ ions in the mixtures co-existing other metal ions, i.e., Hg2+ ions. Particularly, the reversible fluorometric and colorimetric sensing assays with GSH-passivated AgNCs could be expected for copper ions. Subsequently, the application feasibility of GSH-passivated AgNCs for the fluorescence-trackable imaging of live cells and tissues was demonstrated towards the analysis of Cu2+ ions in the complicated media like blood.
Results
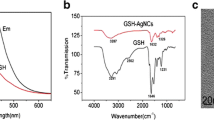
AgNCs were firstly synthesized and passivated with GSH in the presence of DHLA. The resulting GSH-passivated AgNCs were then characterized by the FT-IR spectra (Figure S1), taking GSH and DHLA as the controls. As shown in Figure S1, GSH-passivated AgNCs could present clearly the 1574.1 cm−1 band of carboxyl groups (from DHLA or GSH) and the 3496.3 cm−1 band of amine groups (from GSH), thus confirming the presence of DHLA and GSH. Furthermore, the S-H band (2489.5 cm−1) might not be observed apparently for GSH-passivated AgNCs, implying that AgNCs were covered with the thiol groups-existing DHLA and GSH by the formation of Ag-S bonds. Moreover, AgNCs prepared by using the common template of DHLA might display the selective response to Hg2+ ions by fluorescence quenching (Fig. 1A), as also confirmed elsewhere7. However, one can note from Fig. 1B that upon the GSH passivation the AgNCs could show the specific ion recognition changing from Hg2+ ions to Cu2+ ions. Importantly, the simultaneous fluorimetric and colorimetric assays for Cu2+ ions could thus be expected as demonstrated afterwards.
The fluorometric and colorimetric detection mechanism and procedure for Cu2+ ions is schematically illustrated in Fig. 2A. The addition of Cu2+ ions could markedly quench (“turn off”) the red fluorescence of GSH-passivated AgNCs together with a yellow color change via the GSH-Cu2+ interaction. Herein, GSH of GSH-passivated AgNCs may chelate with Cu2+ ions in molar ratio 2:1 via its carboxyl and amine groups22,23,24, attaining the preferentially stable tetragonal geometry of Cu2+-GSH complex25, as schematically described in Fig. 2B. As a result, the Cu2+-induced specific aggregation of GSH-passivated Ag NCs could undergo resulting in the fluorescence quenching and a decrease in the UV-vis absorbance. It is worth pointing out that the fluorescence quenching of GSH-passivated AgNCs might be partly attributed to the energy transfer between GSH-passivated AgNCs and Cu2+ ions that possess the strong absorbance in the range of red light8. Moreover, the fluorescence lifetime of GSH-passivated AgNCs might not be significantly changed in the presence and absence of Cu2+ ions (data not shown). However, an obvious change in the UV-vis absorbance spectra could be observed for GSH-passivated AgNCs in the presence and absence of Cu2+ ions (Fig. 3A). These evidences suggested that Cu2+ ions might conduct the static fluorescence quenching of GSH-passivated AgNCs26. In addition, the lost fluorescence of the resultant GSH-passivated AgNCs could be restored by introducing a Cu2+ chelating agent, i.e., ethylenediaminetetraacetate (EDTA) with the considerably high Cu2+-binding affinity21, to release the GSH-bound Cu2+ ions from the surface of GSH-passivated AgNCs.
Schematic illustration of (A) the reversible fluorometric and colorimetric detection mechanism and procedure of GSH-passivated AgNCs toward the fluorimetric and colorimetric assays for Cu2+ ions (insert: the photographs of corresponding products) and (B) the chelating interaction between Cu2+ ions and GSH of GSH-passivated AgNCs.
Figure S2 manifests the quantitative comparison of the fluorescence spectra and UV-vis spectra for the EDTA-enabled reversible responses of GSH-passivated AgNCs to Cu2+ ions. As expected, the lost fluorescence (Figure S2A) and UV-vis absorbance (Figure S2B) could be restored over 90% of their initial values by using EDTA. Of note, the emissive fluorescence peaks (650 nm) of the GSH-passivated AgNCs could display only a change in the fluorescence intensity after adding Cu2+ ions, so did the UV-vis absorbance peaks of AgNCs (330, 425 and 500 nm). In contrast, the UV-vis absorption peak (250 nm) of GSH of GSH-passivated AgNCs could show a red shift upon the addition of Cu2+ ions (Figure S2B). Again, the above phenomenon confirms that the Cu2+-induced optical change of GSH-passivated AgNCs should be attributed to the Cu2+-GSH binding. Moreover, the topological structures of the GSH-passivated AgNCs in the absence and presence of Cu2+ ions were characterized by transmission electron microscopy (TEM) imaging (Fig. 3B,C). It was observed that GSH-passivated AgNCs could be well dispersed in the aqueous media with the uniform particle size (Fig. 3B). Once Cu2+ ions (0.50 μM) were introduced, they could get aggregation with the greatly increased particle size (Fig. 3C), so that their average hydrodynamic diameter could change from about 4.3 nm to about 17.5 nm (Figure S3).
The aqueous stability of GSH-passivated AgNCs was investigated over different time intervals of storage under different ionic strengths in NaCl concentrations (Figure S4). Surprisingly, GSH-passivated AgNCs could retain well their fluorescence intensities up to 1.0 M NaCl (Figure S4A), which otherwise could act as a strong precipitate agent for silver ions, achieving the stable storage for six months (Figure S4B). Accordingly, GSH-passivated AgNCs could survive under the harsh conditions, thus promising the potential applications in some complicated media like blood. Furthermore, the pH-dependent fluorescent responses of GSH-passivated AgNCs to Cu2+ ions were examined (Figure S5A), showing the optimal response at about pH 7.0. In addition, a fast response (about one min) to Cu2+ ions could be obtained for GSH-passivated AgNCs (Figure S5B).
The specific fluorometric and colorimetric responses of GSH-passivated AgNCs to Cu2+ ions were further explored comparing to some common metal ions including Al3+, Fe3+, Mg2+, Co2+, Pb2+, Ca2+, Hg2+, K+, Zn2+, Mn2+, Ba2+, Cr3+ and Ni2+ ions (Fig. 4). Obviously, other metal ions including Hg2+ ions caused no significant change in the fluorescence and UV-vis absorbance of GSH-passivated AgNCs, even at the concentrations of 10-fold higher than that of Cu2+ ions. Furthermore, the investigation was conducted on the fluorescent responses of GSH-passivated AgNCs to Cu2+ ions in the mixtures separately co-existing other metal ions (Figure S6), showing the selective sensing of Cu2+ ions. Therefore, the introduction of GSH could effectively modulate the specific ion recognition of AgNCs from Hg2+ ions to Cu2+ ions, thus facilitating the selective fluorometric and colorimetric assays for Cu2+ ions.
Comparable investigation of (A) fluorometric and (B) UV-vis colorimetric responses of GSH-passivated AgNCs (5.0 μM) to Cu2+ ions (1.0 μM) and other metal ions of Al3+, Fe3+, Mg2+, Co2+, Pb2+, Ca2+, Hg2+, K+, Cu2+, Zn2+, Mn2+, Ba2+, Cr3+ and Ni2+ ions (10 μM), with the corresponding photographs of the products under (top) UV and (bottom) visible light.
Under the optimized conditions, the fluorometric assays for Cu2+ ions with different concentrations were performed using GSH-passivated AgNCs as the probes (Fig. 5A). Accordingly, the fluorescence intensities of GSH-passivated AgNCs could decrease with increasing Cu2+ concentrations. A linear detection range could be achieved for Cu2+ concentrations ranging from 0.00010 to 1.0 μM, with the limit of detection (LOD) of about 0.050 nM, estimated by the 3σ rule. Moreover, the colorimetric analysis of Cu2+ ions was carried out (Fig. 5B). The UV-vis spectra of GSH-passivated AgNCs could change depending on the Cu2+ concentrations. A calibration detection curve was also obtained over the linear concentration range of Cu2+ ions of 0.0010–1.0 μM, with the LOD of about 0.60 nM. In addition, a comparison of LOD for Cu2+ ions was conducted among the GSH-passivated AgNCs-based fluorimetry and those analysis methods reported previously, indicating the developed assay could present the lower LOD (Table S1). In addition, the feasibility of the practical applications of the fluorometic and colorimetric assays were explored for Cu2+ ions spiked in blood with different concentrations (Figure S7), showing the LOD of 0.10 nM and 0.80 nM, respectively. Therefore, the developed analysis strategy could facilitate the simultaneous fluorometric and colorimetric assays for Cu2+ ions in blood with high detection sensitivity.
(A) Typical fluorescent emission spectra (left) and the calibration curve (right) of GSH-passivated AgNCs (5.0 μM) versus Cu2+ ions of different concentrations, with the corresponding photographs of testing solutions under UV light (top). (B) Typical UV-vis spectra (left) and the calibration curve (right) of GSH-passivated AgNCs (5.0 μM) versus Cu2+ ions of different concentrations, with the corresponding photographs of detection solutions under white light (top).
Moreover, cytotoxicity (uptake) studies in vitro level were conducted on GSH-passivated AgNCs for culturing yeast cells, with the data shown in Figure S8. The results showed that GSH-passivated AgNCs could present no significant toxicity to the live cells even at a high concentration. Furthermore, the feasibility of fluorescence imaging for yeast cells was also demonstrated in the absence and presence of Cu2+ ions, with the images shown in Figure S9. One can note that the GSH-passivated AgNCs with red fluorescence could light up the yeast cells, of which the fluorescence might be turned off after Cu2+ ions were introduced into the media of cell culture. Furthermore, GSH-passivated AgNCs were employed for the fluorescence-trackable imaging for the live muscle tissues separately from the back and leg parts of a mouse in narcosis, with the typical images shown in Fig. 6. It was observed clearly from the dark-field images that the muscle tissues, which might present no fluorescence (Fig. 6B), could display strong red fluorescence after being treated with GSH-passivated AgNCs (Fig. 6D). In contrast, the injection of Cu2+ ions into the live mouse muscle tissues pretreated with GSH-passivated AgNCs could gradually fade out their red fluorescence (Fig. 6F). These evidences indicated that the GSH-passivated AgNCs, which possess the powerful red fluorescence, good biocompatibility and high aqueous stability, could be tailored for the fluorescence-trackable imaging of live cells and tissues towards the analysis of Cu2+ ions. The extensive biological applications for trackable biotoxicity study and drug delivery in the complicated media like blood could also be expected.
Microscopy fluorescent images of live mouse muscle tissues before (A,B) and after (C,D) being treated with GSH-passivated AgNCs (20 μM) in the bright field (A,C) and dark field (B,D,E) the overlay image of (C,D,F) the image of live mouse muscle tissues treated with GSH-passivated AgNCs (20 μM) containing Cu2+ ions (10 μM) in dark field. The measurement conditions were detailed in the Experimental.
In summary, AgNCs were successfully synthesized and passivated by GSH ligands, so that the specific ion recognition was modulated from Hg2+ ions to Cu2+ ions that could trigger the aggregation of nanoclusters toward the fluorescence quenching. The obtained GSH-passivated AgNCs could display the powerful red fluorescence and high aqueous stability. Meantime, a rational change of UV-vis yellow absorbance spectra of GSH-passivated AgNCs could be obtained depending on the Cu2+ levels. Moreover, the Cu2+-induced loss of fluorescence and UV-vis absorbance of GSH-passivated AgNCs could be efficiently restored by using the Cu2+ chelating agent of EDTA. A simultaneous and reversible fluorometric and colorimetric analysis protocol using GSH-passivated AgNCs has thereby been proposed for probing Cu2+ ions with high detection sensitivity and selectivity. Also, the application feasibility of fluorescence-trackable imaging for live cells and tissues was verified using GSH-passivated AgNCs as the fluorescent probes. The results indicate that GSH-passivated AgNCs could not only facilitate the selective analysis of the Cu2+ ions in complicated media, but also allow for the biological imaging for live cells and tissues, where the background interferences from the endogenous fluorescent species could be efficiently minimized. Such a ligand passivation route may open a new door for modulating the specific ion recognition of noble metal NCs toward the huge applications of the useful optical probes in the functional material synthesis, ion sensing, biological imaging and ion-adsorption separation of biological and chemical interests.
Methods
Materials and Instruments
Glutathione (GSH), α-lipoic acid (LA), silver nitrate (AgNO3), sodium hydroxide, sodium borohydride, copper nitrate (Cu (NO3)2), sodium chloride, ethylenediaminetetraacetate (EDTA), yeast cells, thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (Beijing, China). Yeast peptone dextrose agar (YPD) was obtained from Aladdin. The blood samples were kind provided by the local hospital. All of the chemicals were of analytical grade and all glass containers were cleaned by aqua regia and ultrapure water.
Fluorescence spectrophotometer (F-7000, Hitachi, Japan), UV-3600 spectrophotometer (Shimadzu, Japan), Fourier transform infrared spectrophotometer (FT-IR, Thermo Nicolet Nexus 470FT, USA) and Transmission electron microscopy (TEM, Tecnai G20, FEI, USA) operated at 100 kV were employed to characterize the products of AgNCs in the absence and presence of Cu2+ ions. The hydrodynamic diameters of AgNCs before and after Cu2+ treatment were measured comparably by dynamic light scattering (DLS) with a Zetasizer Nano ZS (Malvern Instruments, UK) setup equipped with a helium-neon laser (λ = 632.8 nm, 4.0 mW). Tissue and cell imaging was observed using the fluorescent inverted microscope (Olympus, IX73-DP80, Japan).
Synthesis of GSH-passivated Ag nanoclusters
Typically, the synthesis of GSH-passivated AgNCs was conducted by adding 125 μL AgNO3 (20 mM) and 150 μL GSH (50 mM) into 5.0 mL ultrapure water at room temperature by magnetic stirring. Then, NaOH (1.0 M) was used to adjust pH to 9.0. At the same time, 5.0 mg LA was mixed with pure sodium borohydride in the molar ratio of LA:NaBH4 = 4:1 and this was stirred well until a clear solution was observed. Here, LA was first reduced in water to form soluble dihydrolipoic acid (DHLA). Then, the prepared DHLA solution was immediately added into the silver mixture by vigorously stirring for one min. Subsequently, a slightly excessive sodium borohydride solution was added dropwise into the mixture. After stirring for 20 min, the mixture was further incubated for 1.5 h at room temperature. After the dialysis and purification, the resulting GSH-passivated AgNCs were stored at 4 °C in the fridge. In addition, the general AgNCs were synthesized according to the procedure reported previously7.
Fluorimetric and colorimetric analysis
Fluorimetric and colorimetric assays with GSH-passivated AgNCs for Cu2+ ions were conducted by the following steps. Typically, an aliquot of GSH-passivated AgNCs were dispersed in the buffer (pH 7.0). Then, a desirable amount of Cu2+ ions with different concentrations was added to be mixed and further incubated for 5 min. Subsequently, the fluorimetric and colorimetric measurements were performed to record the changes of the fluorescence intensities and UV absorbances of the sensing reactions. Moreover, the experiments for the restoring fluorescence of GSH-passivated AgNCs were carried out by using GSH-passivated AgNCs (5.0 μM), Cu2+ ions (0.80 μM) and EDTA (1.6 μM). In addition, the fluorimetic and colorimetric detections were conducted accordingly for Cu2+ ions spiked in blood samples with different concentrations.
Fluorescence-trackable imaging of live cells and tissues
The cytotoxicity tests were firstly performed. An aliquot of yeast cells (1 × 105) in 50 μL buffer was seeded into each of the testing wells on a 96-well plate. After the culture overnight, 50 μL of GSH-passivated AgNCs (0–14.0 μM) or GSH-passivated AgNCs containing Cu2+ ions were separately introduced into the testing wells to be treated for 2 h. Then, an aliquot of 10 μL of MTT solution was added into each of the wells, followed by the incubation at 37 °C for 4 h. Furthermore, an aliquot of 100 μL of solubilization solution containing 10% SDS and 0.010 M HCl was separately added to dissolve the purple crystals by incubating for 12 h. Subsequently, the optical density readings were taken at 595 nm to record the viabilities of yeast cells so cultured using a plate reader.
The fluorescence-trackable imaging was performed for yeast cells. The live cells were firstly centrifugated at 8000 rpm for 5.0 min and then washed with 0.85% NaCl. Following that, the cell samples were diluted and spread on the YPD agar plates to be incubated overnight at 37 °C. After washing for several times, an aliquot of GSH-passivated AgNCs (10 μM) was injected and incubated at 37 °C for 1 h, followed by washing twice. The resulting cell mixtures were separately dropped onto the slides to be imaged separately in the light and dark fields using the fluorescent inverted microscope. Furthermore, an aliquot of Cu2+ ions (5.0 μM) was injected to the mixtures to be incubated at 37 °C for 1 h. Subsequently, the fluorescence imaging of the resulting samples of yeast cells was conducted accordingly.
Moreover, the fluorescence-trackable imaging was carried out for the live muscle tissues separately from the back and leg parts of a mouse in narcosis. An aliquot of GSH-passivated AgNCs (20 μM) was separately injected into the muscle tissues to be treated for 30 min. Then, the fluorescent imaging was conducted for the resulting muscle tissues spread on the slides, taking the ones without GSH-passivated AgNCs as the controls. Furthermore, an aliquot of Cu2+ ions (10 μM) were injected into the live muscle tissues pre-treated with GSH-passivated AgNCs. After being treated further for 1 h, the fluorescent imaging was finally conducted according to the same procedure above.
Additional Information
How to cite this article: Sun, Z. et al. Silver Nanoclusters with Specific Ion Recognition Modulated by Ligand Passivation toward Fluorimetric and Colorimetric Copper Analysis and Biological Imaging. Sci. Rep. 6, 20553; doi: 10.1038/srep20553 (2016).
References
Choi, S., Dickson, R. M. & Yu, J. Developing luminescent silver nanodots for biological applications. Chem. Soc. Rev. 41, 1867–1891 (2012).
Shang, L., Dong, S. & Nienhaus, G. U. Ultra-small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today. 6, 401–418 (2011).
Peyser, L. A., Vinson, A. E., Bartko, A. P. & Dickson, R. M. Photoactivated fluorescence from individual silver nanoclusters. Science. 291, 103–106 (2001).
Sun, Z. et al. High-throughput colorimetric assays for mercury (II) in blood and wastewater based on the mercury-stimulated catalytic activity of small silver nanoparticles in a temperature-switchable gelatin matrix. Chem. Commun. 50, 9196–9199 (2014).
Liu, H. et al. Rapid sonochemical synthesis of highly luminescent non-toxic AuNCs and Au@ AgNCs and Cu (II) sensing. Chem. Commun. 47, 4237–4239 (2011).
Dou, X. et al. Lighting up thiolated Au@Ag nanoclusters via aggregation-induced emission. Nanoscale. 6, 157–161 (2014).
Adhikari, B. & Banerjee, A. Facile synthesis of water-soluble fluorescent silver nanoclusters and HgII sensing. Chem. Mater. 22, 4364–4371 (2010).
Shang, L. & Dong, S. Silver nanocluster-based fluorescent sensors for sensitive detection of Cu (II). J. Mater. Chem. 18, 4636–4640 (2008).
Yeh, H. C. et al. A DNA– Silver Nanocluster Probe That Fluoresces upon Hybridization. Nano Lett. 10, 3106–3110 (2010).
Liu, J. et al. Sensitive and selective detection of Hg2+ and Cu2+ ions by fluorescent Ag nanoclusters synthesized via a hydrothermal method. Nanoscale. 5, 10022–10028 (2013).
Lan, G. Y., Huang, C. C. & Chang, H. T. Silver nanoclusters as fluorescent probes for selective and sensitive detection of copper ions. Chem. Commun. 46, 1257–1259 (2010).
Letelier, M. E. et al. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem. Biol. Interact. 151, 71–82 (2005).
Gaetke, L. M. & Chow, C. K. Copper toxicity, oxidative stress and antioxidant nutrients. Toxicology. 189, 147–163 (2003).
Cui, L., Wu, J., Li, J., Ge, Y. & Ju, H. Electrochemical detection of Cu2+ through Ag nanoparticle assembly regulated by copper-catalyzed oxidation of cysteamine. Biosens. Bioelectron. 55, 272–277 (2014).
Niu, X., Xu, D., Yang, Y. & He, Y. Ultrasensitive colorimetric detection of Cu2+ using gold nanorods. Analyst. 139, 2691–2694 (2014).
Guo, C. L. & Irudayaraj, J. Fluorescent Ag clusters via a protein-directed approach as a Hg (II) ion sensor. Anal. Chem. 8, 2883–2889 (2011).
Zhang, N. et al. Rapid, selective and ultrasensitive fluorimetric analysis of mercury and copper levels in blood using bimetallic gold-silver nanoclusters with “silver effect”-enhanced red fluorescence. Anal. Chem. 86, 11714–11721 (2014).
Baruwati, B., Polshettiwar, V. & Varma, R. S. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chemistry. 11, 926–930 (2009).
Yuan, X. et al. Glutathione-protected silver nanoclusters as cysteine-selective fluorometric and colorimetric probe. Anal. Chem. 85, 1913–1919 (2013).
Chen, W., Tu, X. & Guo, X. Fluorescent gold nanoparticles-based fluorescence sensor for Cu2+ ions. Chem. Commun. 13, 1736–1738 (2009).
Muhammed, M. H. et al. Luminescent quantum clusters of gold in bulk by albumin-induced core etching of nanoparticles: metal ion sensing, metal-enhanced luminescence and biolabeling. Chem. Eur. J. 16, 10103–10112 (2010).
Fang, C. & Zhou, X. Voltammetry and EQCM investigation of glutathione monolayer and its complexation with Cu2+. Electroanalysis. 15, 1632–1638 (2003).
Liu, J. M., Wang, H. F. & Yan, X. P. A gold nanorod based colorimetric probe for the rapid and selective detection of Cu2+ ions. Analyst. 136, 3904–3910 (2011).
Chen, Z. et al. Sensitive and selective detection of glutathione based on resonance light scattering using sensitive gold nanoparticles as colorimetric probes. Analyst. 137, 3132–3137 (2012).
Sigel, H. & Martin, R. B. Coordinating properties of the amide bond: stability and structure of metal ion complexes of peptides and related ligands. Chem. Rev. 82, 385–426 (1982).
Fu, Y., Li, H., Hu, W. & Zhu, D. Fluorescence probes for thiol-containing amino acids and peptides in aqueous solution. Chem. Commun. 25, 3189–3191 (2005).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 21375075 and 21573126) and the Taishan Scholar Foundation of Shandong Province, P. R. China.
Author information
Authors and Affiliations
Contributions
H.W. conceived the project and designed the experiments. Z.S. conducted the main experiments, data analysis and wrote the paper. S.L. performed the synthesis and fluorescence tests. Y.J., Y.Q. and L.Z. did the characterization tests of UV-vis absorbance and fluorescence. L.X. and J.L. assisted the characterization of TEM imaging and FT-IR spectra. W.Q. contributed to the tissue and cell imaging.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, Z., Li, S., Jiang, Y. et al. Silver Nanoclusters with Specific Ion Recognition Modulated by Ligand Passivation toward Fluorimetric and Colorimetric Copper Analysis and Biological Imaging. Sci Rep 6, 20553 (2016). https://doi.org/10.1038/srep20553
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20553
- Springer Nature Limited
This article is cited by
-
Fluorescent Gold Nanoclusters for Selective Detection of Dopamine in Cerebrospinal fluid
Scientific Reports (2017)
-
C-phycocyanin from Spirulina maxima as a Green Fluorescent Probe for the Highly Selective Detection of Mercury(II) in Seafood
Food Analytical Methods (2017)
-
Rapid and sensitive detection of ketamine in blood using novel fluorescence genosensor
Analytical and Bioanalytical Chemistry (2017)