Abstract
A potent algicidal bacterium isolated from Lake Taihu, Chryseobacterium sp. strain GLY-1106, produces two algicidal compounds: 1106-A (cyclo(4-OH-Pro-Leu)) and 1106-B (cyclo(Pro-Leu)). Both diketopiperazines showed strong algicidal activities against Microcystis aeruginosa, the dominant bloom-forming cyanobacterium in Lake Taihu. Interestingly, these two algicidal compounds functioned synergistically. Compared with individual treatment, combined treatment with cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) significantly enhanced algicidal activity, accelerated the increase in intracellular reactive oxygen species (ROS) levels in M. aeruginosa and further decreased the activities of antioxidases, effective quantum yield and maximal electron transport rate of M. aeruginosa. The results also showed that the algicidal characteristics of cyclo(4-OH-Pro-Leu) are distinct from those of cyclo(Pro-Leu). Cyclo(4-OH-Pro-Leu) mainly interrupted the flux of electron transport in the cyanobacterial photosynthetic system, whereas cyclo(Pro-Leu) mainly inhibited the activity of cyanobacterial intracellular antioxidases. A possible algicidal mechanism for the synergism between cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) is proposed, which is in accordance with their distinct algicidal characteristics in individual and combined treatment. These findings suggest that synergism between algicidal compounds might be used as an effective strategy for the future control of Microcystis blooms.
Similar content being viewed by others
Introduction
Cyanobacterial blooms have become a worldwide aquatic environmental problem due to increasing water eutrophication and climate change1,2. Lake Taihu, the third largest freshwater lake in China, with a surface area of 2338 km2 3, is no exception. With the discharge of nutrient-rich municipal and industrial wastewater and overapplication of chemical fertilizers around the basin, Lake Taihu has undergone severe eutrophication, leading to frequent large scale outbreaks of cyanobacterial blooms4. Microcystis is the dominant bloom-forming cyanobacterium in Lake Taihu5; Microcystis spp. blooms have also attracted global concern due to their adverse influence on ecology and health4,6.
Nutrient input control is the ultimate strategy for the control of harmful algal blooms (HABs)7. Meanwhile, numerous other strategies have been used to manage HABs. Given that the application of chemical (eg. copper sulfate and sodium hypochlorite) and physical methods (eg. clays and flocculants) has been challenged due to the costs and secondary pollution8, biomanipulation technologies have received particular attention because of their potential efficacy and eco-friendliness8,9. Algicidal bacteria are considered to be associated with the regulation and termination of blooms in some lakes10,11. Recently, many algicidal bacteria have been isolated9,12,13,14 and some of the corresponding algicidal substances have also been purified and identified, including pigments14,15, diketopiperazines16,17, proteins18,19 and other complex compounds9,20.
In our previous work, five algicidal bacterial strains were isolated from Lake Taihu and four algicidal substances were separated and identified from the algicidal bacteria: pigment (isatin)14, diketopiperazines (cyclo(Pro-Gly) and cyclo(Pro-Val))16 and hydroquinone17. Recently, a potent algicidal strain, Chryseobacterium sp. strain GLY-1106, was isolated from Lake Taihu, which produces two algicidal compounds. More interestingly, the combination of these two algicidal substances showed an enhanced algicidal effect, indicating that synergism existed between the two compounds. Accordingly, this study focused on the possible mechanism involved in the observed algicidal synergism of these two algicidal compounds.
Results
Isolation and identification of algicidal bacteria
A total of 212 isolates were obtained from surface water samples collected from Meiliang Bay of Lake Taihu in October 2012. Among these strains, approximately 8% (17 isolates) exhibited strong algicidal activity (A) (A ≥ 90%, t = 6 days) against Microcystis aeruginosa 9110. The strain GLY-1106, which had the strongest algicidal activity (A = 98.9%, t = 6 days) (see supplementary Fig. S1a online), was selected for further in-depth investigation. This strain formed shiny, compact yellow colonies with entire edges (see supplementary Fig. S1b online) and the morphological examination (see supplementary Fig. S1c online) via transmission electron microscopy revealed that strain GLY-1106 was a rod-shaped (0.5 to 1.0 by 3.0 to 4.0 μm) bacterium. Bacterial growth occurred under both aerobic and anaerobic conditions, but did not take place at temperatures of ≥37 °C. The Gram-negative isolate hydrolyzed casein and was phosphatase-, catalase- and oxidase-positive, but did not show arginine-, ornithine- and lysine- decarboxylase or phenylalanine deaminase activity. Acid was formed from D-glucose, but no gas was formed. The strain did not produce hydrogen sulfide from triple-sugar iron agar. When the 16S rRNA gene sequence was compared with the sequence available in the GenBank database, the strain GLY-1106 most closely resembled the type strain Chryseobacterium piscium LMG 23089T (99% identity, GenBank accession number NR_042410). Most of the physiological properties of strain GLY-1106 were similar to those of C. piscium (see supplementary Table S1 online). However, some properties were different: in contrast to C. piscium, the isolate GLY-1106 utilized maltose and D-glucose rather than acetic acid and it also tested negative for phenylalanine deaminase activity and did not grow in the presence of 5% NaCl. By combining the physiological and biochemical phenotypic characteristics and the results of phylogenetic analysis (see supplementary Fig. S2 online), the newly isolated strain was designated as Chryseobacterium sp. strain GLY-1106. The 16S rRNA gene sequence data of strain GLY-1106 have been submitted to the GenBank database under accession number KP342510. This strain has been deposited in the China General Microbiological Collection Center (CGMCC) under accession number CGMCC-9953.
Algicidal mode of Chryseobacterium sp. strain GLY-1106
As shown in Supplementary Fig. S3, the algicidal activity of the strain GLY-1106 culture (A = 98.9%, t = 6 days) was slightly higher than that of its cell-free filtrate (A = 90.7%, t = 6 days) and much higher (P < 0.01) than that of its re-suspended washed cells (A = 37.5%, t = 6 days). No significant (P > 0.05) difference was observed between the algicidal activities of cell-free filtrate and heat-treated cell-free filtrate. These results indicated that strain GLY-1106 exhibited algicidal activity mainly by producing heat-stable extracellular algicidal compounds to attack cyanobacterial cells indirectly.
Algicidal range of Chryseobacterium sp. strain GLY-1106
The algicidal activity of strain GLY-1106 was evaluated according to its efficiency in inhibiting on the dominant bloom-forming cyanobacterial species in Lake Taihu and other cyanobacterial and algal species (Table 1). Similar to most of the cyanobacterial species tested, especially those isolated from Meiliang Bay in Lake Taihu, strain GLY-1106 exhibited strong algicidal effects. After 6 days of co-culture, strain GLY-1106 showed the highest algicidal activity (A = 98.8%) against M. aeruginosa 9110 among all the cyanobacterial species tested. In addition, the strain GLY-1106 also exhibited an algicidal effect on Chlamydomonas sp. BS3 (A = 95.2%, t = 6 days), a eukaryotic algal strain from Lake Taihu.
Extraction and purification of the algicidal compounds
The ethyl acetate extract of the cell-free culture filtrate of strain GLY-1106 showed strong algicidal activity against M. aeruginosa 9110 on cyanobacterial lawns. Following silica gel chromatography of the extract, one fraction which exhibited algicidal activity was obtained from the effluent of the chromatographic column. When the fraction had been collected and applied to semi-preparative high performance liquid chromatography (HPLC), two primary fractions in the effluent from HPLC exhibited algicidal activity on cyanobacterial lawns, i.e. fractions A (retention time (RT): 20.0–21.5 min) and B (RT: 34.8–37.2 min) (see supplementary Fig. S4 online). Fractions A and B were subsequently treated separately by further-purification HPLC. Finally, two purified substances exhibiting algicidal activity, i.e. 1106-A (RT: 58.2–61.0 min, see supplementary Fig. S5 online) and 1106-B (RT: 65.2–74.5 min, see supplementary Fig. S6 online), were obtained from the effluent of further-purification HPLC, respectively.
Identification of the algicidal compounds
The molecular structures of 1106-A and 1106-B were resolved by high-resolution electrospray ionization mass spectrometry (ESI-MS), electron ionization mass spectrometry (EI-MS) and nuclear magnetic resonance (NMR).
The positive-mode high-resolution ESI-MS spectrum showed the mass charge ratio (m/z) of 1106-A (M+H)+ to be 227.1390 (see supplementary Fig. S7 online), which suggested a molecular formula of C11H18N2O3. The EI-MS spectrum of 1106-A indicated that ions at 86 and 170 corresponded to molecule (M)-C6H6NO3 and M-C4H8 ions, respectively (see supplementary Fig. S8 online). Compared with EI-MS spectrum of 1106-A, there was not an EI-MS spectrum of the compound in the GC/MS library with a higher similar index (SI, SI > 800). The NMR data for 1106-A (see supplementary Fig. S9 online) were in agreement with those previously reported for 7-hydroxy-3-isobutyl-hexahydro-pyrrolo[1,2-a]pyrazine-1,4-dione21,22.
The positive-mode high-resolution ESI-MS spectrum showed the (M+H)+ of 1106-B at m/z 211.1441 (see supplementary Fig. S10 online), which suggested a molecular formula of C11H18N2O2. The EI-MS spectrum of 1106-B indicated that ions at 70 and 154 corresponded to molecule (M)-C7H10NO2 and M-C4H8 ions, respectively (see supplementary Fig. S11a online). The EI-MS spectrum of 1106-B was similar (SI > 960) to that of hexahydro-3-(2-methylpropyl)-pyrrolo[1,2-a]pyrazine-1,4-dione (molecular weight (MW): 210, molecular formula: C11H18N2O2) in the GC/MS library (see supplementary Fig. S11b online). The NMR data for 1106-B (see supplementary Fig. S12 online) were in agreement with those previously reported for hexahydro-3-(2-methylpropyl)-pyrrolo[1,2-a]pyrazine-1,4-dione23.
In summary, 1106-A and 1106-B were identified as 7-hydroxy-3-isobutyl- hexahydro-pyrrolo[1,2-a]pyrazine-1,4-dione (abbreviated as “cyclo(4-OH-Pro-Leu)”) (Fig. 1a) and hexahydro-3-(2-methylpropyl)-pyrrolo[1,2-a]pyrazine-1,4-dione (“cyclo(Pro-Leu)”) (Fig. 1b), respectively.
Dynamics of the cellular density of strain GLY-1106, concentration of algicidal compounds and cyanobacterial biomass during the algicidal process
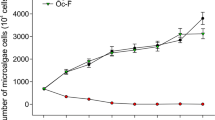
As shown in Fig. 2, the cellular density of strain GLY-1106 was significantly positively related to the concentration of algicidal compounds (cyclo(4-OH-Pro-Leu): r = 0.975, P < 0.01; cyclo(Pro-Leu): r = 0.994, P < 0.01) and significantly negatively related to the cyanobacterial biomass (r = −0.948, P < 0.01) in general during the algicidal process of strain GLY-1106 against M. aeruginosa 9110. After 12 h of co-cultivation, when the cellular density of strain GLY-1106 increased to 8.3 × 106 CFU ml–1 in the co-culture of M. aeruginosa 9110 and strain GLY-1106, the algicidal compounds were still undetectable and the algicidal activities were not observed. After 24 h of co-cultivation, when the cellular density strain GLY-1106 increased to 5.1 × 107 CFU ml–1, only the algicidal compounds cyclo(Pro-Leu) was detected, at a concentration of approximately 0.19 μg ml–1, leading to weak algicidal activity. When the cellular density of strain GLY-1106 increased to 1.2 × 108 CFU ml–1 on the 2nd day, the concentrations of cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) reached 0.51 and 0.77 μg ml–1, respectively, resulting in a sharp drop in the cellular density of M. aeruginosa 9110 to 43.8% of the control (without inoculation of strain GLY-1106). After 6 days of co-cultivation, M. aeruginosa 9110 almost disappeared, the cellular density of strain GLY-1106 increased to 7.36 × 108 CFU ml–1 and the concentration of cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) rised to 5.03 and 6.92 μg ml–1, respectively.
Dynamics of Microcystis aeruginosa 9110, strain GLY-1106 and algicidal compounds in the co-culture during algicidal process.
(a) Growth curve of M. aeruginosa 9110 inoculated with algicidal strain GLY-1106 and without strain GLY-1106 (control). Initial concentration of strain GLY-1106 in the cocultures was about 2.0 × 106 CFU ml–1. (b) Variations of the concentration of algicidal compounds and cell density of strain GLY-1106 in the coculture of Microcystis aeruginosa 9110 and strain GLY-1106. The vertical bar represents the standard deviation of triplicate samples.
EC50 values of cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu)
As shown in Fig. 3, cyclo(4-OH-Pro-Leu) generally showed a stronger algicidal effect than cyclo(Pro-Leu). The EC50 values (concentrations for 50% of maximal algicidal effect) of cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) calculated from the dose response curve were 1.26 and 2.70 μg ml–1, respectively.
Cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) exhibited a synergistic algicidal effect on M. aeruginosa 9110
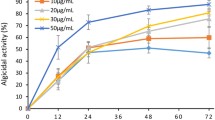
As shown in Fig. 3, individual treatment with cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) at a concentration of 0.4 or 0.8 μg ml–1 showed a weak or intermediate algicidal activity against M. aeruginosa 9110. Therefore, the concentration of 0.4 μg ml–1 (cyclo(4-OH-Pro-Leu)) plus 0.4 μg ml–1 (cyclo(Pro-Leu)) was used in the combined treatment. The results in Fig. 4 indicate that cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) exhibited synergistic algicidal effects against M. aeruginosa 9110. Individual treatment with cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) showed obvious algicidal activity after 6 and 9 h of exposure, respectively, while the combined treatment showed obvious algicidal activity after 3 h of exposure. Moreover, the algicidal activity of the combined treatment was significantly (P < 0.01) higher than that obtained in the individual treatments at the same time point during the algicidal process. After 48 h of exposure, the algicidal activity of individual treatment with cyclo(4-OH-Pro-Leu) at 0.4 μg ml–1 and 0.8 μg ml–1 was 21.4% and 33.7%, respectively and the algicidal activity of individual treatment with cyclo(Pro-Leu) at 0.4 μg ml–1 and 0.8 μg ml–1 was 10.2% and 19.5%, respectively. However, the algicidal activity of the combined treatment (0.4 μg ml–1 cyclo(4-OH-Pro-Leu) plus 0.4 μg ml–1 cyclo(Pro-Leu)) reached 56.3% after 48 h, which was 1.67, 2.63, 2.89 and 5.51 times higher than that of the individual treatment with cyclo(4-OH-Pro-Leu) (0.8 μg ml–1), cyclo(4-OH-Pro-Leu) (0.4 μg ml–1), cyclo(Pro-Leu) (0.8 μg ml–1) and cyclo(Pro-Leu) (0.4 μg ml–1), respectively and was 1.78 times higher than the sum of the activity following individual treatment with cyclo(4-OH-Pro-Leu) (0.4 μg ml–1) and cyclo(Pro-Leu) (0.4 μg ml–1).
Cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) synergistically enhanced the damage to cyanobacterial physiology
To further investigate the underlying mechanism of the synergistic algicidal effects of cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) on M. aeruginosa 9110, the physiological responses of the cyanobacterium to the individual and combined treatments were determined, including the changes in reactive oxygen species (ROS) level, malondialdehyde (MDA) content, antioxidase activity, effective quantum yield (Φe) and maximal electron transport rate (rETRmax).
As shown in Fig. 5a, the intracellular ROS levels in M. aeruginosa 9110 after individual treatment with cyclo(4-OH-Pro-Leu) at 0.4 μg ml–1 and 0.8 μg ml–1 reached a maximum after 12 h and 9 h of exposure, respectively and these two peak ROS levels were 1.28 and 1.36 times higher, respectively, than those of the control at the same time points. The ROS levels after individual treatment with cyclo(Pro-Leu) at 0.4 μg ml–1 and 0.8 μg ml–1 showed a slowly increase with time. After 48 h of exposure, the ROS levels in M. aeruginosa 9110 after individual treatment with cyclo(Pro-Leu) (0.4 μg ml–1) and cyclo(Pro-Leu) (0.8 μg ml–1) were 1.21 and 1.32 times higher than that of the control, respectively. The intracellular ROS level in M. aeruginosa 9110 with the combined treatment of cyclo(4-OH-Pro-Leu) (0.4 μg ml–1) plus cyclo(Pro-Leu) (0.4 μg ml–1) exhibited a greater and continuous tendency to increase compared with that after the individual treatments and the increased ROS levels after the combined treatment were significantly (P < 0.01) higher than those after the individual treatments at each time point during the incubation process. After 48 h of exposure, the increased ROS level following the combined treatment was 2.26 times higher than the sum of the individual treatments with cyclo(4-OH-Pro-Leu) (0.4 μg ml–1) and cyclo(Pro-Leu) (0.4 μg ml–1).
As an indicator of lipid peroxidation24, the MDA content in M. aeruginosa 9110 (Fig. 5b) showed a continuous increase in all the treatments. During the algicidal process, the increased MDA content with the combined treatment (0.4 μg ml–1 cyclo(4-OH-Pro-Leu) plus 0.4 μg ml–1 cyclo(Pro-Leu)) was significantly (P < 0.01) higher than those in the individual treatments. After 48 h of exposure, the increased MDA content after the combined treatment was 1.75 times higher than the sum of the individual treatments with cyclo(4-OH-Pro-Leu) (0.4 μg ml–1) and cyclo(Pro-Leu) (0.4 μg ml–1).
As shown in Fig. 6, the response of the antioxidant enzyme system to the algicidal compounds cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) was obviously different during the algicidal process. The activities of superoxide dismutase (SOD) (Fig. 6a) following the individual treatment with cyclo(4-OH-Pro-Leu) showed an activating effect compared with the control and generally exhibited a tendency first to increase then to descend. The SOD activities after the individual treatment with cyclo(4-OH-Pro-Leu) (0.4 μg ml–1) and cyclo(4-OH-Pro-Leu) (0.8 μg ml–1) reached the highest levels after 15 and 12 h of exposure, respectively. However, the activities of SOD (Fig. 6a) following the individual treatment with cyclo(Pro-Leu) showed an inhibitory effect compared with the control and generally exhibited a tendency to decline throughout the algicidal process. The activities of SOD with the combined treatment (0.4 μg ml–1 cyclo(4-OH-Pro-Leu) plus 0.4 μg ml–1 cyclo(Pro-Leu)) showed a much greater reducing trends with time. In particular, the activities of SOD after the combined treatment decreased dramatically after 6 h of exposure and were significantly lower than those following the individual treatments. After 48 h of exposure, the activities of SOD with the combined treatment were only 21.3% of those in the control. During the algicidal process, the activities of catalase (CAT) (Fig. 6b) and peroxidase (POD) (Fig. 6c) varied and resembled those of SOD (Fig. 6a). Following individual treatment with cyclo(4-OH-Pro-Leu), the activities of SOD (cyclo(4-OH-Pro-Leu) (0.4 μg ml–1): r = 0.722, P < 0.01; cyclo(4-OH-Pro-Leu) (0.8 μg ml–1): r = 0.611, P < 0.05) and POD (cyclo(4-OH-Pro-Leu) (0.4 μg ml–1): r = 0.556, P < 0.05; cyclo(4-OH-Pro-Leu) (0.8 μg ml–1): r = 0.558, P < 0.05) were significantly and positively correlated with ROS levels. However, the activities of these three antioxidases were significantly and negatively (r = –1.000, P < 0.01, in all cases) correlated with ROS levels following individual treatment with cyclo(Pro-Leu) and the combined treatment.
As shown in Fig. 7a,b, the Φe and rETRmax following all treatments showed a tendency to decline during the algicidal process. The Φe and rETRmax following individual treatment with cyclo(4-OH-Pro-Leu) (0.8 μg ml–1) or cyclo(4-OH-Pro-Leu) (0.4 μg ml–1) decreased gradually with time and were 60.9% and 39.7%, or 75.6% and 49.93% of the control after 48 h of exposure, respectively. The Φe and rETRmax following individual treatment with cyclo(Pro-Leu) (0.8 μg ml–1) or cyclo(Pro-Leu) (0.4 μg ml–1) began to decrease after 12 h and were 89.3% and 80.5%, or 94.6% and 88.2% of the control after 48 h of exposure, respectively. The Φe and rETRmax after the combined treatment (0.4 μg ml–1 cyclo(4-OH-Pro-Leu) plus 0.4 μg ml–1 cyclo(Pro-Leu)) decreased at a higher rate and were lower than those following individual treatments at each time point during the algicidal process. In particular, after 6 h, with the highest and continuous increase in ROS level in the combined treatment (Fig. 5a), these two photosynthetic parameters decreased markedly compared with those following individual treatments and the control. After 48 h of exposure, the Φe was 34.1% of the control, while rETRmax was almost 0% with the combined treatment. During the algicidal process, the decrease in rETRmax was greater than that of Φe with the combined treatment and individual treatment with cyclo(4-OH-Pro-Leu), but not individual treatment with cyclo(Pro-Leu). In addition, both Φe and rETRmax were significantly (r = −1.000, P < 0.01) and negatively correlated with the ROS level following the combined treatment.
Discussion
Cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) are diketopiperazines, which are the simple peptide derivatives. Diketopiperazines constitute a large class of small molecules synthesized by a broad range of microorganisms which show several useful biological properties25 and act as antibacterial compounds26, antitumor and immunosuppressive agents27 and so on. The biosynthesis of diketopiperazines is thought to be catalyzed either by nonribosomal peptide synthetases or by cyclodipeptide synthases28. Cyclo(Pro-Gly), produced by Shewanella sp. strain Lzh-214 and Stenotrophomonas sp. strain F617 and cyclo(Pro-Val), produced by Bacillus sp. strain Lzh-516, showed algicidal activity against M. aeruginosa. Compared with cyclo(Pro-Gly) and cyclo(Pro-Val), cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) showed the similar or stronger algicidal activities against M. aeruginosa. To the best of our knowledge, the cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) isolated from Chryseobacterium spp. in this study are newly discovered algicidal substances.
Chemicals with similar structures often show distinct biological properties. Xiao and colleagues6 isolated a pair of chiral flavonolignans from barley straw and found that, after short-term exposure, one of the chiral flavonolignans caused significant damage to cyanobacterial cell membranes, but had no influence on the intracellular ROS level, whereas the other chiral flavonolignan induced an increase in intracellular ROS level, but no damage to the cell membrane. In the current study, the difference in the chemical composition between cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) is only one hydroxyl group, but their biological properties are vastly different. Synergism may be a common characteristic of some co-occurring secondary metabolites and a powerful driving force in their evolution29. Synergism occurring naturally has been observed in bacteriocins, which are peptides of ribosomal origin produced by lactic acid bacteria30. The co-produced secondary metabolites of some Actinomycetes act synergistically against either a target microorganism or competitors for nutrients29,31. In the present study, the combination of cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) significantly enhanced the algicidal activity against the bloom-forming M. aeruginosa. To the best of our knowledge, this is the first report of the synergistic algicidal action of diketopiperazine algicidal substances.
ROS are involved in damage to living organisms under environmental stress32. Excessive ROS may cause irreversible oxidative damage to intracellular components, finally leading to cell death33. The reaction centers of the photosystem are the major generation sites of ROS in cells34. During the photosynthesis process in cyanobacteria, chlorophyll a in photosystem II initially captures photons with sufficient energy (λ < 680 nm) and generates electrons; after this, the electrons are transferred via cytochrom bf to photosystem I and are used to produce the NADPH (dihydronicotinamide adenine dinucleotide phosphate)1. The photoproducton of ROS can be enhanced by decreased capacity of the electron transport flux34. On the other hand, superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) are three important antioxidases that can scavenge the excessive ROS and protect cells from damage caused by oxygen free radicals20. During the algicidal process in the present study, individual treatment with cyclo(4-OH-Pro-Leu) caused a much greater decrease in photosynthetic quantum yield and electron transport rate of M. aeruginosa than individual treatment with cyclo(Pro-Leu) and the degree of reduction in the electron transport rate was larger than that in the photosynthetic quantum yield, indicating that cyclo(4-OH-Pro-Leu) may induce excessive production of ROS in M. aeruginosa mainly by interrupting the electron transport flux. In addition, the antioxidase system was activated by individual treatment with cyclo(4-OH-Pro-Leu) during the algicidal process and the activities of SOD, CAT and POD were much higher than those following the individual treatment with cyclo(Pro-Leu). In contrast to cyclo(4-OH-Pro-Leu), individual treatment with cyclo(Pro-Leu) caused a significant decrease in antioxidase activities during the algicidal process, but showed a weak or even negligible influence on photosynthetic quantum yield and electron transport rate, which suggests that cyclo(Pro-Leu) may induce excessive levels of ROS in M. aeruginosa mainly by inhibiting the activities of antioxidases. It can be seen that the algicidal characteristics of cyclo(4-OH-Pro-Leu) are distinct from those of cyclo(Pro-Leu).
Compared with individual treatment with cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu), the combined treatment not only significantly aggravated the antioxidases activities, but also interrupted electron transport flux in the photosystem of M. aeruginosa, resulting in a synergistic algicidal effect. With regard to the marked decrease in the activities of antioxidases in the combined treatment after 6 h of exposure, the oxidative damages induced by the continuous increase of ROS level could play a key role in this phenomenon. Accordingly, the mechanism underlying the synergistic algicidal effect of cyclo(4-OH-Pro-Leu) (mainly inhibiting photosynthesis) and cyclo(Pro-Leu) (mainly inhibiting antioxidase activity) may be as follows: both the photosynthetic capacity and antioxidase activities in cyanobacterial cells are inhibited by the two substances simultaneously, accelerating the disruption of redox homeostasis and the increase in intracellular ROS level and lipid peroxidation; in addition, the higher level of ROS may further accelerate the decline in antioxidase activities35 and photosynthetic capacity36,37, resulting in a greater increase in ROS and more oxidative damage in cyanobacterial cells.
Based on the results of this study, it could be seen that the synergism really exists between some algicidal compounds produced by algicidal bacterium, although there was no such phenomenon found in the previous studies on the algicidal compounds derived from algicidal bacteria. Therefore, determining whether or not algicidal synergism exists among more known algicidal compounds derived from algicidal bacteria and exploiting the collaborative mechanism would be of great significance in the future. In addition, synergism between algicidal compounds might be used as an effective strategy for the future control of Microcystis blooms.
Methods
Cyanobacterial and algal strains and cultivation conditions
Microcystis aeruginosa 9110 (maintained as a unialgal axenic culture, Collection No. CGMCC 9118 in the China General Microbiological Culture Collection Center), Synechococcus sp. BN60 (CGMCC 9117), Chlorophyta sp. B1, Oscillatoria sp. BN35 and Chlamydomonas sp. BS3 were isolated from Meiliang Bay in Lake Taihu13,14. Chroococcus sp. FACHB-191, Microcystis aeruginosa PCC7806 and Microcystis viridis FACHB-979 were purchased from the Freshwater Algae Culture Collection at the Institute of Hydrobiology (FACHB-collection), Chinese Academy of Science, China. All the above-mentioned cyanobacterial and algal strains were maintained in sterilized BG11 medium14. Throughout the study, all the cyanobacterial and algal cultures were incubated at 25 °C under 40 μmol photons (m2·sec)–1 and a 12 h: 12 h (light: dark) cycle13.
Chemicals
Standard cyclo(4-OH-Pro-Leu) (7-hydroxy-3-isobutyl-hexahydro-pyrrolo[1,2-a]pyrazine-1,4-dione) and cyclo(Pro-Leu) (hexahydro-3-(2-methylpropyl)-pyrrolo[1,2-a]pyrazine-1,4-dione) (purity ≥ 99%), which were used for the dose response bioassays and the investigation of the synergistic algicidal effect on M. aeruginosa 9110, were synthesized by G L Biochem Ltd. (Shanghai, China). The chemicals used for the preparation of all culture media and chemical analysis were purchased from Sigma-Aldrich (St. Louis, MO, USA), if not specifically mentioned.
Isolation and identification of algicidal bacteria, determination of algicidal mode and range of strain GLY-1106
Water samples were collected from the surface water of the Taihu Ecosystem Research Station (31°24′N, 120°13′E) in hypertrophic Meiliang Bay located in the northeast area of Lake Taihu, using a Ruttner Standard Water Sampler, in October 2012. The water samples collected were immediately transferred into sterile bottles and transported to the laboratory in a mini-icebox. Algicidal bacteria were subsequently isolated from the water samples and identified as described in Supplementary Information online. The methods used for determination of the algicidal mode and range of strain GLY-1106 are detailed in Supplementary Information online.
Calculation of algicidal activity and survival rate
The algicidal activity (A, %) and survival rate (Rs, %) were calculated on the basis of the following equations13,14: A = (1 − Dt-treatment/Dt-control) × 100 and Rs(%) = Dt-treatment/Dt-control × 100, where Dt-treatment and Dt-control are the cell densities of cyanobacteria in the treatment and control, respectively; t (day or hour) represents the inoculation time. Cell densities of M. aeruginosa (cells ml−1) were determined using a hemocytometer under a light microscope (BH-2, Olympus, Japan; Magnification: × 400). For other cyanobacteria, chlorophyll a (chl-a) concentrations (mg l−1) representing the equivalent cell densities were quantified spectrophotometrically using the acetone method38.
Separation, purification and identification of bacterial algicidal compounds and quantitative analysis of algicidal compounds secreted from strain GLY-1106 by UPLC-MS during the algicidal process
The procedures used for separation, purification and identification of algicidal compounds and for quantitative analysis of algicidal compounds, were as described in our previous work14 with slight modifications as described in Supplementary Information online.
Dose response bioassays of cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) against M. aeruginosa 9110
The dose response bioassays were performed with an initial cyanobacterial density of 1 × 107 cells ml−1 according to the method by Li and colleagues14. Stock solutions of standard cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) were prepared by dissolving them in sterile distilled water. An aliquot (0.1 ml) of each stock solution (0.01, 0.02, 0.04, 0.06, 0.08, 0.1, 0.15, 0.2, 0.25, 0.3, 0.4, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, or 10.0 mg ml−1) of standard cyclo(4-OH-Pro-Leu) or cyclo(Pro-Leu) was added to a 9.9-ml culture of M. aeruginosa 9110 to yield a final concentration of 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2, 2.5, 3, 4, 5, 10, 20, 30, 40, 50, or 100 μg ml–1, respectively. In addition, control (without addition of algicidal compounds) was prepared using an equal amount of sterile distilled water instead of the standard algicidal compound solution. After 24 h of incubation under cyanobacterial growth conditions, the corresponding survival rate (Rs) of M. aeruginosa 9110 was examined. The EC50 values were calculated from the relevant dose response curves by probit analysis39.
Synergistic effect of cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) against M. aeruginosa 9110
To examine whether there was a synergistic effect between cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu), combined treatment and corresponding individual treatments with these two algicidal compounds were conducted with an initial cyanobacterial density of 1 × 107 cells ml−1. Briefly, in the combined treatment, 0.5-ml aliquots of stock solutions (0.08 mg ml−1) of standard cyclo(4-OH-Pro-Leu) and cyclo(Pro-Leu) were added to a 99-ml culture of M. aeruginosa 9110 (99-ml culture of M. aeruginosa 9110, 0.5 ml of cyclo(4-OH-Pro-Leu) and 0.5 ml of cyclo(Pro-Leu); total, 100 ml), respectively. In the individual treatments, an aliquot (1 ml) of each stock solution (0.04 or 0.08 mg ml–1) of standard cyclo(4-OH-Pro-Leu) or cyclo(Pro-Leu) was added to a 99-ml culture of M. aeruginosa 9110 to yield a final concentration of 0.4 or 0.8 μg ml–1, respectively. In addition, 1-ml sterile distilled water was added to a 99-ml culture of M. aeruginosa 9110 as the control (without addition of algicidal compounds). The cyanobacterial cultures in the treatment groups and control were incubated for 48 h under cyanobacterial growth conditions. During the incubation period, aliquots from both treatment groups and the control were sampled to determine algicidal activities and other relevant physiological indices at the following time points: 0, 3rd, 6th, 9th, 12th, 15th, 18th, 24th and 48th hour.
Flow cytometry-based analyses on the intracellular ROS level
Flow cytometric measurements were carried out using a FACSAria II flow cytometer (BD Biosciences, USA) equipped with Diva software for data acquisition. FlowJo software (Tree Star, USA) was used for data analysis. For each sample of cyanobacterial cells, 20,000 events were collected by flow cytometer. Cyanobacterial intracellular ROS formation was determined at the single-cell level following the method described by Wang and colleagues40 and the membrane-permeable dye 2′,7′-dichlorodihydroflurescein diacetate (No. D6883, Sigma) was used as a probe. When the probe molecules enter cells, they may be transformed into 2′,7′-dichlorodihydrofluorescein (H2DCF) by intracellular esterase. Once the intracellular ROS generated, H2DCF would be converted into highly fluorescent 2′,7′-dichlorofluorescein (DCF). Therefore, we determined the fluorescence intensity (FI) of DCF by flow cytometry to indicate the extent of ROS generation. Approximately 106 cells in the treatment groups and control were harvested by centrifugation at 3,000 × g for 10 min, washed twice with sterile phosphate-buffered saline (PBS) solution (50 mM, pH 7.0) and re-suspended in 1 ml PBS (50 mM, pH 7.0). The cyanobacterial suspensions containing 10 μM H2DCF-DA were incubated in the dark at 25 °C for 60 min and then washed twice with sterile fresh PBS solution. The stained cells were then analyzed by flow cytometry. Changes in ROS levels as compared with the control were evaluated using the following formula40,41:

Determination of antioxidase activity and lipid peroxidation
Cyanobacterial cells were pelleted by centrifugation at 12,000 × g and 4 °C for 20 min and washed twice with PBS (50 mM, pH 7.8). After the pellets had been resuspended in PBS, the cells were disrupted using an Ultrasonic Cell Disruption System (NingBo Scientiz Biotechnological Co., Ltd, China) (200 W, ultrasonic time: 2 s; rest time: 8 s, 30 times) in an ice-bath. The supernatant was then collected by centrifugation for 20 min at 12,000 × g and 4 °C and used to investigate the physiological changes, including antioxidase activity, total protein and malondialdehyde (MDA, a byproduct of lipid peroxidation) content. Total protein content, concentration of MDA and the activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) were determined using Total Protein Quantitative assay kit (No. A045-2), Malondialdehyde assay kit (No. A003-1), Total Superoxide Dismutase (T-SOD) assay kit (No. A001-1), Catalase assay kit (No. A007-1) and Peroxidase assay kit (No. A084-3) purchased from Nanjing Jiancheng Institute, China, respectively, according to the manufacturer’s instructions.
Determination of photosynthetic performance
The photosynthetic performance of photosystem II was determined using a PHYTO-PAM phytoplankton analyzer (Walz, Germany) following the method described by Ou and colleagues42. A 5-ml sample of the suspension was analyzed immediately after harvesting. When a sample was acclimated to the light in its environment, effective quantum yield (Φe) can be calculated as follows:

where Fs and Fm' are the corresponding light-acclimated steady-state and maximum fluorescence, respectively and △F is the difference between Fm' and Fs. The photosynthetic parameter (Φe) is an approximation of the fraction of absorbed energy used for photochemistry in the total energy at a specific time and is therefore dimensionless43. The other photosynthetic parameter (rETRmax), which indicates the maximum photosynthetic capacity with the unit of μmol m–2 s–1 44, was calculated following the method described by Ralph and Gademann45. In practice, these parameters can be obtained directly from the PHYTO-PAM analyzer.
Data analysis
The data in this study were obtained from three replicates and are presented as means±standard deviation. One-way analysis of variance (ANOVA) was carried out using SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA). Comparisons between the means were conducted using Duncan’s Multiple Range Test. The correlation analysis and probit analysis were conducted using SPSS v 20.0 (IBM Corp., Armonk, NY, USA).
Additional Information
How to cite this article: Guo, X. et al. Synergistic algicidal effect and mechanism of two diketopiperazines produced by Chryseobacterium sp. strain GLY-1106 on the harmful bloom-forming Microcystis aeruginosa. Sci. Rep. 5, 14720; doi: 10.1038/srep14720 (2015).
References
Wu, Y. et al. Allelopathic control of cyanobacterial blooms by periphyton biofilms. Environ. Microbiol. 13, 604–615 (2011).
Lewitus, A. J. et al. Harmful algal blooms along the North American west coast region: history, trends, causes and impacts. Harmful Algae 19, 133–159 (2012).
Wu, Q. L. et al. Submersed macrophytes play a key role in structuring bacterioplankton community composition in the large, shallow, subtropical Taihu Lake, China. Environ. Microbiol. 9, 2765–2774 (2007).
Otten, T. G., Xu, H., Qin, B., Zhu, G. & Paerl, H. W. Spatiotemporal patterns and ecophysiology of toxigenic Microcystis blooms in Lake Taihu, China: implications for water quality management. Environ. Sci. Technol. 46, 3480–3488 (2012).
Ye, W. J. et al. Temporal variability of cyanobacterial populations in the water and sediment samples of Lake Taihu as determined by DGGE and real-time PCR. Harmful Algae 10, 472–479 (2011).
Xiao, X. et al. A pair of chiral flavonolignans as novel anti-cyanobacterial allelochemicals derived from barley straw (Hordeum vulgare): Characterization and comparison of their anti-cyanobacterial activities. Environ. Microbiol. 16, 1238–1251 (2014).
Lin, S. et al. Characterization of an algicidal bacterium Brevundimonas J4 and chemical defense of Synechococcus sp. BN60 against bacterium J4. Harmful Algae 37, 1–7 (2014).
Anderson, D. M. Turning back the harmful red tide. Nature 388, 513–514 (1997).
Zheng, X. et al. A marine algicidal actinomycete and its active substance against the harmful algal bloom species Phaeocystis globosa. Appl. Microbiol. Biotechnol. 97, 9207–9215 (2013).
Rashidan, K. K. & Bird, D. F. Role of predatory bacteria in the termination of a cyanobacterial bloom. Microb. Ecol. 41, 97–105 (2001).
Zhang, P. et al. The dynamics of the water bloom-forming Microcystis aeruginosa and its relationship with biotic and abiotic factors in Lake Taihu, China. Ecol. Eng. 47, 274–277 (2012).
Kim, Y. S., Lee, D. S., Jeong, S. Y., Lee, W. J. & Lee, M. S. Isolation and characterization of a marine algicidal bacterium against the harmful raphidophyceae Chattonella marina. J. Microbiol. 47, 9–18 (2009).
Tian, C. et al. Isolation, identification and characterization of an algicidal bacterium from Lake Taihu and preliminary studies on its algicidal compounds. J. Environ. Sci. 24, 1823–1831 (2012).
Li, Z. et al. A freshwater bacterial strain, Shewanella sp. Lzh-2, isolated from Lake Taihu and its two algicidal active substances, hexahydropyrrolo[1,2-a]pyrazine-1,4-dione and 2, 3-indolinedione. Appl. Microbiol. Biotechnol. 98, 4737–4748 (2014).
Yang, F. et al. Isolation and characterization of an algicidal bacterium indigenous to Lake Taihu with a red pigment able to lyse Microcystis aeruginosa. Biomed. Environ. Sci. 26, 148–154 (2013).
Li, Z. H., Geng, M. X. & Yang, H. Algicidal activity of Bacillus sp. Lzh-5 and its algicidal compounds against Microcystis aeruginosa. Appl. Microbiol. Biotechnol. 99, 981–990 (2015).
Lin, S., Geng, M., Liu, X., Tan, J. & Yang, H. On the control of Microcystis aeruginosa and Synechococccus species using an algicidal bacterium, Stenotrophomonas F6 and its algicidal compounds cyclo-(Gly-Pro) and hydroquinone. J. Appl. Phycol. in press (2015).
Chen, W. M., Sheu, F. S. & Sheu, S. Y. Novel L-amino acid oxidase with algicidal activity against toxic cyanobacterium Microcystis aeruginosa synthesized by a bacterium Aquimarina sp. Enzyme Microb. Technol. 49, 372–379 (2011).
Wang, B. X. et al. A marine bacterium producing protein with algicidal activity against Alexandrium tamarense. Harmful Algae 13, 83–88 (2012).
Luo, J. F. et al. Isolation and identification of algicidal compound from Streptomyces and algicidal mechanism to Microcystis aeruginosa. PloS one 8, e76444 (2013).
Sun, S. et al. A novel natural product from the fermentation liquid of marine fungus Trichoderma atroviride G20-12. Asian J. Trad. Med. 4, 123–127 (2009).
Kalinovskaya, N. I., Kalinovsky, A. I., Romanenko, L. A., Dmitrenok, P. S. & Kuznetsova, T. A. New angucyclines and antimicrobial diketopiperazines from the marine mollusk-derived actinomycete Saccharothrix espanaensis An 113. Nat. Prod. Commun. 5, 597–602 (2010).
Yan, P. S. et al. Cyclo(L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl. Environ. Microbiol. 70, 7466–7473 (2004).
Qian, H. et al. Effects of glufosinate on antioxidant enzymes, subcellular structure and gene expression in the unicellular green alga Chlorella vulgaris. Aquat. Toxicol. 88, 301–307 (2008).
Ortiz-Castro, R. et al. Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc. Natl. Acad. Sci. USA 108, 7253–7258 (2011).
Fdhila, F., Vazquez, V., Sánchez, J. L. & Riguera, R. DD-diketopiperazines: antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 66, 1299–1301 (2003).
Kanoh, K. et al. Antitumor activity of phenylahistin in vitro and in vivo. Biosci. Biotechnol. Biochem. 63, 1130–1133 (1999).
Belin, P. et al. The nonribosomal synthesis of diketopiperazines in tRNA-dependent cyclodipeptide synthase pathways. Nat. Prod. Rep. 29, 961–979 (2012).
Challis, G. L. & Hopwood, D. A. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl. Acad. Sci. USA 100, 14555–14561 (2003).
Garneau, S., Martin, N. I. & Vederas, J. C. Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 84, 577–592 (2002).
McCafferty, D. G., Cudic, P., Yu, M. K., Behenna, D. C. & Kruger, R. Synergy and duality in peptide antibiotic mechanisms. Curr. Opin. Chem. Biol. 3, 672–680 (1999).
He, Y. Y. & Hader, D. P. Involvement of reactive oxygen species in the UV-B damage to the cyanobacterium Anabaena sp. J. Photochem. Photobiol. B-Biol. 66, 73–80 (2002).
Apel, K. & Hirt, H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 (2004).
Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141, 391–396 (2006).
Alzolibani, A. A. Preferential recognition of hydroxyl radical-modified superoxide dismutase by circulating autoantibodies in patients with alopecia areata. Ann. Dermatol. 26, 576–583 (2014).
Hideg, E. & Schreiber, U. Parallel assessment of ROS formation and photosynthesis in leaves by fluorescence imaging. Photosynth. Res. 92, 103–108 (2007).
Ma, Z. L. & Gao, K. S. Spiral breakage and photoinhibition of Arthrospira platensis (Cyanophyta) caused by accumulation of reactive oxygen species under solar radiation. Environ. Exp. Bot. 68, 208–213 (2010).
Welschmeyer, N. A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 39, 1985–1992 (1994).
Zhang, C., Yi, Y. L., Hao, K., Liu, G. L. & Wang, G. X. Algicidal activity of Salvia miltiorrhiza Bung on Microcystis aeruginosa-towards identification of algicidal substance and determination of inhibition mechanism. Chemosphere 93, 997–1004 (2013).
Wang, Z., Li, J., Zhao, J. & Xing, B. Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter. Environ. Sci. Technol. 45, 6032–6040 (2011).
Hong, Y. et al. Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 91, 262–269 (2009).
Ou, H., Gao, N., Deng, Y., Qiao, J. & Wang, H. Immediate and long-term impacts of UV-C irradiation on photosynthetic capacity, survival and microcystin-LR release risk of Microcystis aeruginosa. Water Res. 46, 1241–1250 (2012).
Genty, B., Briantais, J.-M. & Baker, N. R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta-Gen. Subj. 990, 87–92 (1989).
Ihnken, S., Eggert, A. & Beardall, J. Exposure times in rapid light curves affect photosynthetic parameters in algae. Aquat. Bot. 93, 185–194 (2010).
Ralph, P. J. & Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 82, 222–237 (2005).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21477077 & No. 21277089).
Author information
Authors and Affiliations
Contributions
X.G. and H.Y. designed experiments. X.G., X.L. and J.P. performed the experiments. X.G., X.L. and H.Y. analyzed the data. X.G. and H.Y. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Guo, X., Liu, X., Pan, J. et al. Synergistic algicidal effect and mechanism of two diketopiperazines produced by Chryseobacterium sp. strain GLY-1106 on the harmful bloom-forming Microcystis aeruginosa. Sci Rep 5, 14720 (2015). https://doi.org/10.1038/srep14720
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14720
- Springer Nature Limited
This article is cited by
-
Higher resistance of a microcystin (MC)-producing cyanobacterium, Microcystis, to the submerged macrophyte Myriophyllum spicatum
Environmental Science and Pollution Research (2023)
-
Ecological damage of submerged macrophyte Myriophyllum spicatum by cell extracts from microcystin (MC)- and non-MC-producing cyanobacteria, Microcystis
Journal of Oceanology and Limnology (2022)
-
Monitoring and control methods of harmful algal blooms in Chinese freshwater system: a review
Environmental Science and Pollution Research (2022)
-
Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from planting water of Pontederia cordata
Environmental Science and Pollution Research (2022)
-
Biology and applications of co-produced, synergistic antimicrobials from environmental bacteria
Nature Microbiology (2021)