Abstract
This novel method enables specific measurement of the activation of hybrid receptors formed between the Insulin Receptor (IR) and the Insulin-like Growth Factor 1 Receptor (IGF1R). These hybrid receptors are present in tissues and cell lines expressing both IR and IGF1R. It is therefore challenging to separate the homodimer and hybrid receptor activation properties. This ELISA method enabled fast and quantitative measurements of activated hybrid receptors. The hybrid receptor specificity is obtained from a combination of two specific antibodies for IGF1R and for an IR tyrosine phosphorylation site. The specificity was shown by immunoprecipitations and Western blot analysis. IR exists as two splice variants; consequently, two splice variants of hybrid receptors can be expressed. It is reported here that both splice variants of insulin/IGF1 receptor hybrids are activated by IGF1 with >20-fold higher potency than insulin.
Similar content being viewed by others
Introduction
Hybrid receptors are formed between the Insulin Receptor (IR) and Insulin-like Growth Factor 1 Receptor (IGF1R) and have been detected in all tissues and cell lines that express both receptor types1,2,3. IR and IGF1R are highly homologous tetramers consisting of two alpha-beta subunits linked by disulfide bonds and containing tyrosine kinase in the membrane-spanning beta subunit. They are formed in the endoplasmic reticulum before they reach the plasma membrane4. The hybrid receptors consist of one IR alpha-beta subunit linked to one IGF1R alpha-beta subunit2. Two splice variants of the hybrid receptors exist because IR is expressed either with (IR-B) or without 12 amino acids encoded by exon 11 (IR-A)5. Hence, IR-A/IGF1R (Hybrid-A) and IR-B/IGF1R (Hybrid-B) hybrid receptors can be formed. The 12 amino acids are located at the C-terminal of the extra-cellular IR alpha subunit in the vicinity of the ligand-binding domain6. Ligands for IR and IGF1R are the hormones insulin, Insulin-like Growth Factor 1 (IGF1) and Insulin-like Growth Factor 2 (IGF2). These hormones affect metabolism and growth in normal physiology, but also influence the progression of cancer and diabetes2,7,8.
The biological function of the hybrid receptors is still unclear, although several studies on their effect on insulin sensitivity are published. More specifically, by increasing the relative expression level between IGF1R and IR, cells have been shown to lose their insulin sensitivity because hybrid receptors bind insulin with low affinity9,10. Studies on rodent skeletal muscle with overexpression of dominant negative IGF1R resulted in insulin resistance because of the incorporation of IR into the non-insulin responsive hybrid receptor11. The negative effect of hybrid receptor formation on insulin sensitivity has also been shown in human diabetic tissues. Hybrid receptors are present in human pre-adipocytes12 and their number is increased in adipose tissue from patients with type 2 diabetes compared with non-diabetic controls13. In another insulin-sensitive tissue, human skeletal muscle, the number of hybrid receptors is also increased in patients with type 2 diabetes14 and correlates negatively with the insulin sensitivity15. In the vascular endothelium, insulin has an important role in maintaining vascular health by stimulating release of the vasoactive molecule nitric oxide (NO), with beneficial mitigating effects on inflammation, thrombosis, vascular tone and oxidative stress16. Similar to the findings in insulin-responsive tissue, the formation of hybrid receptors can reduce the insulin responsiveness in the vascular endothelium by increasing the ratio between IGF1R and IR17. Human platelets also have IR receptors but their insulin responsiveness is weak, likely due to the relatively higher expression of IGF1R, leading to hybrid receptor formation18. It is not only in diabetes that insulin/IGF1 receptor hybrid formation is important: in cancer cells, an increase in hybrid receptor expression is detected and the IR splice variant in the hybrid receptor is often IR-A2,19,20. IGF1 and IGF2 bind preferentially to IR-A with up to a 10-fold difference between the IR splice variants, albeit that IGF1 binding is weak10,21. The high affinity of IGF2 for IR-A is important in cancer cells because these often express the IR-A isoform as well as IGF2, creating an autocrine loop2.
IGF1 and IGF2 have been shown to bind to hybrid receptors with high affinity whereas human insulin binds with low affinity9,10. However, there is some discrepancy in the literature since one group reports that insulin can bind to Hybrid-A with high affinity22. Since insulin and IGFs are physiologically significant hormones, it is important to establish to what degree the hybrid receptors are activated as a response to binding these hormones. Although several studies18,23,27, report hybrid activation, the assays used have not been hybrid receptor specific, whereas in this study, hybrid receptor activation was studied specifically. This was achieved by using a specific IGF1R antibody followed by an antibody binding tyrosine-phosphorylated IR at position Y1334. This epitope is not conserved in IGF1R so is expected to be specific for the IR half in the hybrid receptor. This is confirmed by immunoprecipitations and Western blot analysis in this study. The combination of antibodies was adapted to an enzyme-linked immunosorbent assay (ELISA), enabling the study of hybrid receptors by ligands and their relative potency. The method only detects hybrid receptor activation and not homodimer IR or IGF1R activation.
Results
Specificity of insulin/IGF1 receptor hybrid activation assay
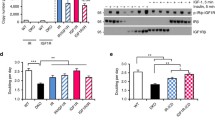
In order to study whether the antibody combination of IGF1R 24–31 and IR pY1334 could be used in a specific hybrid receptor-activation assay, immunoprecipitation and SDS-PAGE analysis were first performed (Fig. 1). Lysates were prepared from baby hamster kidney (BHK) cells co-expressing IGF1R and IR-A, which were either unstimulated or stimulated with 1, 10 or 100 nM insulin (lanes 1–4) or IGF1 (lanes 5–8) for 30 minutes. Control cells overexpressing only IR-A (lanes 9, 10, 13 and 14) or IGF1R (lanes 11 and 12) were stimulated with 100 nM insulin or IGF1. Two blots for analysis were made; one for immunoprecipitated receptors (Fig. 1a, b and c) and one for the cell lysates that were the input for the immunoprecipitations (Fig. 1d, e and f). In this study, the blots were reprobed, taking advantage of differences in migration within the gel (i.e. the alpha subunit at 120 kDa and the beta subunit at 97 kDa). Hybrid activation was detected in the immunoprecipitated cell lysate with the specific IGF1R antibody 24–31 and Western blot analysis using the specific antibody IR pY1334 (Fig. 1a, lanes 1–8). There was a tendency for the sensitivity to IGF1 to be greater (Fig. 1a, lanes 5–8) than to insulin (Fig. 1a, lanes 1–4). This method is a specific hybrid receptor-activation assay, since co-expression of IR and IGF1R was needed to detect the IR pY1334 of immunoprecipitated IGF1R. The method distinguishes between homo and hetero dimer receptors activation. No signal was detected from the cell line, overexpressing only IGF1R (Fig. 1a, lanes 11 and 12) and from cells expressing only IR-A, no IGF1R was immunoprecipitated (Fig. 1b, lanes 9 and 10), hence IR could not be immunoprecipitated as part of a hybrid receptor. This also proved the specificity of the IGF1R 24–31 antibody. The antibody IR pY1334 can detect activated IR since it gave a strong signal when IR was immunoprecipitated with the IR-specific antibody 83–7 after stimulation with insulin or IGF1 (Fig. 1a, lanes 13 and 14). In the lysates from BHK cells overexpressing both IR and IGF1R (Fig. 1d, lanes 1–4), tyrosine phosphorylation of IR pY1334 was detected even at low doses of insulin. This signal was primarily from homodimer IR rather than hybrid receptors because this high sensitivity to insulin was not detected after immunoprecipitating with IGF1R antibody 24–31 (Fig. 1a, lanes 1–4). The difference in the dose response to IGF1 and insulin was not caused by different expression levels or variations in immunoprecipitations since equal levels of IGF1R were detected in all relevant lanes (Fig. 1b and e) at 120 kDa with the IGF1R alpha subunit antibody N-20. The Western blot was reprobed and the bleed through from the pY1334 probing from panel A or D was detected at 97 kDa. Similarly, an equal IR level was present in all relevant lanes detected, with C-19 antibody recognising the 97 kDa beta subunit (Fig. 1c and f). The bleed through from the N-20 blot was detected at 120 kDa.
Specific insulin/IGF1 receptor hybrid activation assay.
Western blot analysis of immunoprecipitations (a, b and c) with 24–31 (lanes 1–12) and with 83–7 (lanes 13 and 14) on lysates from BHK cells expressing IR-A and IGF1R (lanes 1–8), IR-A (lanes 9, 10, 13 and 14) or IGF1R (lanes 11 and 12). Cell lysate analysis (d, e and f). The blots were probed with IR pY1334 (a and d), IGF1R N-20 (b and e) and IR C-19 (c and f). Cells were either unstimulated (lanes 1 and 5) or stimulated with 1, 10 or 100 nM insulin (lanes 2, 3 and 4), with IGF1 (lanes 6, 7 and 8), with 100 nM insulin (lanes 9, 11 and 13) or with 100 nM IGF1 (lanes 10, 12 and 14) for 30 minutes at 37°C. The data shown is one representative of four independent experiments. BHK, baby hamster kidney; IGF1, Insulin-like Growth Factor 1; IGF1R, Insulin-like Growth Factor 1 Receptor; IR, Insulin Receptor; IP, ImmunoPrecipitation.
Specific ELISA for insulin/IGF1 receptor hybrid activation
In order to quantitatively compare the activation of insulin/IGF1 receptor hybrids by ligands, an ELISA was developed using the antibody combination described above. The specific hybrid ELISA was created by coating 96 well plates with IGF1R specific 24–31 antibody, with detection using IR pY1334 antibody. The secondary anti-rabbit antibody coupled with horseradish peroxidase ensured a readout of 450 nm after addition of the reagent. Representative concentration–response curves from activation of Hybrid-A and Hybrid-B are shown in Fig. 2a and b. The two splice variants of the hybrid receptors were significantly more responsive to IGF1 than insulin with respective EC50 values for Hybrid-A and Hybrid-B hybrid receptors of 6 ± 3 nM and 12 ± 2 nM with IGF1 and 342 ± 121 nM and 325 ± 88 nM with insulin (Table 1). The maximal insulin response on tyrosine phosphorylation of IR pY1334 reached 93% and 88% of the maximal response of IGF1 for Hybrid-A and Hybrid-B, respectively. In order to test the assay on a widely used cell line, the human Hepatoma cell line (HepG2) was chosen because it is known to contain IR, IGF1R and insulin/IGF1 receptor hybrids23. The activation of hybrid receptors was detected with the ELISA, with EC50 1.9 ± 1.0 nM and 42.4 ± 12.3 nM after IGF1 and insulin stimulation, respectively (Table 1).
ELISA measurements of specific insulin/IGF1 receptor hybrid activation.
Activation of the insulin/IGF1 receptor hybrid Hybrid-A (a) and Hybrid-B (b) measured by detection in ELISA of tyrosine phosphorylation of IRY1334. Concentration–response curves for insulin (Δ) and IGF1 ( ) stimulation (see Methods).
) stimulation (see Methods).
Discussion
It is important to distinguish between IR and IGF1R homodimer and insulin/IGF1 receptor hybrid activation. This novel method enables specific and quantitative measurements of the hybrid receptors in cell lines and tissues by the use of specific IGF1R and IR PTyr antibodies. It has been difficult to measure hybrid activation specifically because hybrid receptors will only be present when IR and IGF1R are co-expressed, hence homodimer receptors will also be present. Studies of IR and IGF1R activation use immunocapturing of IR or IGF1R with specific antibodies, followed by analysis with antibodies for tyrosine phosphorylation; alternatively, it can be done the other way round. These methods capture both the homo- and heterodimer receptors. Studies have been carried out using hybrid receptors23,27 but, again, they lack an assay that specifically measures hybrid activation. The ability to determine hybrid receptor responses will be important for unravelling the biological functions of hybrids, including their role in cancer. The presence of hybrid receptors is likely to be an important contributor to the lack of success in developing inhibitory monoclonal antibodies against the IGF1R in human clinical trials for cancer therapy24. The IGF1R antibodies (e.g. IMC-A12) were selected on the basis of their lack of cross-reactivity with IR to avoid toxicity such as high blood glucose levels24. Subsequently, it was shown that IMC-A12 could decrease tumour growth in a transgenic mouse model of pancreatic neuroendocrine carcinogenesis when the IR level was decreased, demonstrating that the effect on tumour growth depends on the IR and IGF1R composition25. The decreased tumour growth in this model is likely due to the specific IGF1R antibodies inhibiting the activation of homodimer IGF1R, but not the insulin/IGF receptor hybrid. When both IR and IGF1R are expressed in high levels, many of the receptors will exist as hybrid receptors. Decreasing IR expression will abolish hybrid receptor formation hence IGF1R will mainly exist as homodimer receptors that are responsive to the antibody. Many anti-cancer antibodies are designed to bind to the ligand binding site. The IR ligand binding site consists of Site 1 and Site 26,26. The binding site in the hybrid receptor is altered compared to the homodimers since Site 1 will be made of one half of IR and one half of IGF1R10. Tumours with high levels of IR will have insulin/IGF receptor hybrids and specific IGF1R therapies may therefore inhibit the homodimer IGF1R but not the hybrid receptor. Thus, it is important to study both the IR and IGF1R homodimers as well as their heterodimer receptors.
The specificity of the antibody combination 24–31 and IR pY1334 for hybrid receptor activation was shown by immunoprecipitation and Western blot analysis of lysates from BHK cells overexpressing IR, IGF1R or both. There are two specific tyrosine residues at the C-terminal end of IR: IRY1328 and 1334. IGF1R has tyrosine at the C-terminal end but the adjacent amino acids are not conserved, giving the specificity for IR. In this report, data have been shown for IR pY1334 only, but the other IR tyrosine phosphorylation site, IR pY1328, gave similar results (data not shown). The human IGF1R homodimer and IGF1/insulin receptor hybrids were immunoprecipitated with the 24–31 antibody. This antibody does not bind well to rat IGF1R (data not shown), so this combination of antibodies will work on some, but not all, species. The antibody combination was transferred to an ELISA, enabling a more precise and quantitative study of the concentration response to insulin and IGF1 stimulation and determination of EC50 values. IGF1 was to elicit a response with 30- to 60-fold higher potency at Hybrid-A and Hybrid B compared with insulin. For both Hybrid-A and Hybrid-B, the maximal response to insulin stimulation reached only 88–93% of that from IGF1 stimulation. This is problematic when comparing the calculated EC50 values; however, it can be concluded that IGF1 was a more potent activator of both hybrid receptors than insulin. Whether there is a biological function for the different maximal level of response needs further investigation.
In order to test a widely used cell line, the HepG2 cell line was chosen. It is a human hepatoma cell line known to have IR, IGF1R and the hybrid receptors23. The challenge of determining whether the homodimer and/or the hetero dimer receptors are activated has been discussed23. The ELISA worked well on this cell line and revealed EC50 values of 1.9 ± 1.0 nM and 42.4 ± 12.3 nM after IGF1 and insulin stimulation, respectively. The EC50 levels are lower than in the BHK cells overexpressing the receptors. This discrepancy could result from variation in several cellular events following receptor stimulation. The receptors are autophosphorylated after stimulation, but they are also dephosphorylated by phosphatases and the receptors are internalised. The balances between these events might vary between cell lines, giving different EC50 values but, importantly, the relative potency between the ligands is of the same order.
There is some disagreement as to whether insulin binds to Hybrid-A with high affinity9,10,22. The results here correlated with the binding data reported earlier, indicating that neither of the two splice variants of the hybrid receptor has a high affinity to insulin, but both have a high affinity to IGF9, as found in studies using tagged receptors10. The results support the potential role of hybrid receptors in reducing the insulin responsiveness of cells by increasing the IGF1R expression and thereby incorporating more IR into hybrid receptors. IR and IGF1R have overlapping downstream signalling cascades2,8, but it is unclear how the hybrid receptors are signalling. Whether they signal as an IR, an IGF1R or a combination of the two needs further investigation and this new method is a step in that direction.
Methods
Materials and cell lines
Human insulin and IGF1 was provided by Novo Nordisk (Bagsvaerd, Denmark). Human Serum Albumin A-1887 obtained from Sigma-Aldrich (St. Louis, MO, USA). Microtiter plate 96 wells LockWell C8, MaxiSorp #446469 from Thermo Fisher Scientific Nunc (Roskilde, Denmark). IGF1R specific antibody 24–3128 and IR specific antibody 83–729 were licensed from Prof Siddle, Cambridge University, UK. The antibody for tyrosine phosphorylated IRY1334 Rabbit PAb Anti-IR (pY1334) #44-809G was purchased from BioSource (Invitrogen, Carlsbad, CA, USA). The antibodies IGF1Rα N-20 (sc-712) and Insulin Rβ C-19 were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). Cell lines BHK (21 TK- from ATCC) were stably transfected with pZEM encoding human IR-A, IR-B, IGF1R, IR-A and IGF1R (BTHK53 + 163-2) or IR-B and IGF1R (BTHK141 + 163-8)9. The HepG2 cell line was purchased from ATCC (HB-8065).
Insulin/IGF1 receptor hybrid activation assays
Activation of insulin/IGF1 receptor hybrids were studied in HepG2 cells and in BHK cells overexpressing human IGF1R and IR-A or IR-B. As control cells, BHK cell lines overexpressing IR-A or IGF1R alone were included. The cells were seeded in 6-well plates for immunoprecipitations or 12-well plates for ELISA and grown until 80–90% confluent. Cells were grown in DMEM (Gibco, Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum, 100 μg/ml penicillin and 100 U/ml streptomycin at 37°C in 5% CO2 humidified atmosphere. Transfected BHK cells were grown in the presence of 1 μM methotrexate. Cells were washed twice with phosphate-buffered saline (PBS) and starved in DMEM containing 0.1% human serum albumin for 1 hour before stimulation with increasing concentrations of insulin or IGF1 for 30 minutes at 37°C. Subsequently, cells were washed three times in ice-cold PBS and snap-frozen by pouring liquid nitrogen into the wells. Cells were lysed in 300 μl (6-well plates) or 100 μl (12-well plates) cell extraction buffer (BioSource, Invitrogen, Life Technologies, Carlsbad, CA, USA) with 1 mM AEBSF (Sigma-Aldrich), 1 mg/ml p-nitrophenyl phosphate (Amresco LLC, OH, USA) and 200 μM Na3VO4. The lysates were pre-cleared by centrifugation at 14,000 g for 10 min at 4°C.
Insulin/IGF1 receptor hybrid activation assay by immunoprecipitation and Western blot analysis was performed by addition of 30 μl of Protein G-Agarose (Roche Diagnostic, Indianapolis, IN, USA) in 300 μl buffer (100 mM Hepes, pH 7.8, 100 mM NaCl, 10 mM MgSO4, 0.02% Tween-20) and 1 μg of the appropriate antibody for 3 hours at 4°C followed by brief centrifugation. The precipitates were washed three times in PBS with 0.02% Tween-20, 200 mM Na3VO4, 1 mM AEBSF and 1 mg/ml p-nitrophenyl phosphate. For gel electrophoresis, the immunoprecipitates were resuspended in reducing loading buffer (Novex, Life Technologies, Carlsbad, CA, USA) with TCEP (Pierce, Thermo Scientific, IL, USA) and the proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Life Technologies). The immunoreactive proteins were visualised using horseradish peroxidase-coupled secondary antibodies (Thermo Scientific) and enhanced chemiluminescence reagents according to the manufacturer's instructions (ECL Select Western Blotting Detection Reagent RPN 2235, GE Healthcare, Amersham).
Insulin/IGF1 receptor hybrid activation assay by ELISA was performed by coating microtiter 96-well plates (Lockwell C8, MaxiSorp) with 50 μl/well 24–31 30 μg/ml in PBS. The plate was washed with 0.05% Tween-20 in PBS and blocked with 0.2% Tween-20 in PBS. After washing with 0.05% Tween-20 in PBS, an appropriate amount of lysate was added and incubated overnight at 4°C. The plate was washed with 0.05% Tween-20 in PBS and incubated for 1 hour with IR antibody pY1334 1:500 dilution in PBS with 0.05% Tween-20. After washing with 0.05% Tween-20 in PBS, the secondary antibody ImmunoPure Antibody Anti-Rabbit IgG (#31460, Thermo Scientific) was diluted 1:5000 in 0.05% Tween-20 in PBS, incubated for 1 hour and washed again in 0.05% Tween-20 in PBS. 100 μl/well TMB One Solution #G7431 (Promega, CA, USA) was added and incubated for 30 minutes. 100 μl/well Stop Solution #50SS03 (Invitrogen, Life Technologies) was added and read on an ELISA-reader on 450 nm. The procedure was performed at room temperature.
Calculation and statistics
Calculation of EC50 values was determined using non-linear regression algorithm with four-parameter logistics in GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Standard deviation was calculated using Microsoft Excel.
References
Bailyes, E. M. et al. Insulin receptor/IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem. J. 327, 209–215 (1997).
Belfiore, A., Frasca, F., Pandini, G., Sciacca, L. & Vigneri, R. Insulin receptor isoform and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 30, 586–623 (2009).
Pandini, G. et al. Insulin and insulin-like growth factor-1 (IGF-I) hybrid receptor overexpression in breat cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin. Cancer Res. 5, 1935–1944 (1999).
Adams, T. E., Epa, V. C., Garrett, T. P. J. & Ward, C. W. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. 57, 1050–1093 (2000).
Mosthaf, L. et al. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 9, 2409–2413 (1990).
De Meyts, P. & Whittaker, J. Structural biology of insulin and IGF1 receptors: implications for drug design. Nat. Rev. Drug Discov. 1, 769–783 (2002).
Girnita, L., Worrall, C., Takahashi, S. –I., Seregard, S. & Girnita, A. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptors (IGF-1R) signaling regulation. Cell. Mol. Life Sci. 71, 2403–2427 (2014).
Boucher, J., Kleinridders, A. & Kahn, C. R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 6, 1–23 (2014).
Slaaby, R. et al. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I (IGF-IR) have low insulin and high IGF-1 affinity irresepctive of the IR splice variant. J. Biol. Chem. 281, 25869–25874 (2006).
Benyoucef, S., Surinya, K. H., Hadaschik, D. & Siddle, K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternative spliced exon11 sequence to ligand binding and receptor activation. Biochem. J. 403, 603–613 (2007).
Fernandez, A. M. et al. Functional inactivation of IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 15, 1926–1934 (2001).
Bäck, K. & Arnqvist, H. J. Changes in insulin and IGF-I receptor expression during differentiation of human preadipocytes. Growth Horm. IGF Res. 19, 101–111 (2009).
Federici, M. et al. Increased abundance of insulin/IGF-I hybrid receptors in adipose tissue from NIDDM patients. Mol. Cell. Endocrinol. 135, 41–47 (1997).
Federici, M. et al. Increased expression of insulin/insulin-like growth factor-I hybrid receptors in skeletal muscle of noninsulin-dependent diabetes mellitus subjects. J. Clin. Invest. 98, 2887–2893 (1996).
Federici, M. et al. Increased abundance of insulin/insulin-like growth factor-I hybrid receptor in skeletal muscle of obese subjects is correlated with in vivo insulin sensitivity. J. Clin. Endocrinol. Metab. 83, 2911–2915 (1998).
Kearney, M. T. Changing the way we think about endothelial cell insulin sensitivity, nitric oxide and the pathophysiology of type 2 diabetes. Diabetes 62, 1386–1388 (2013).
Abbas, A. et al. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes 60, 2169–2178 (2011).
Hunter, R. W. & Hers, I. Insulin/IGF-I hybrid receptor expression on human platelets: consequences for the effect of insulin on platelet function. J. Thromb. Haemost. 7, 2123–2130 (2009).
LeRoith, D. & Roberts, C. T. The insulin-like growth factor system and cancer. Cancer Lett. 195, 127–137 (2003).
Trajkovic-Arsic, M., Kalideris, E. & Siveke, J. T. The role of insulin and IGF system in pancreatic cancer. J. Mol. Endocrinol. 50, 67–74 (2013).
Denley, A. et al. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon11 minus isoform of the IR. Mol. Endocinol. 18, 2505–2512 (2004).
Pandini, G. et al. Insulin/insulin-like growth factor I hybrid receptors have different biological characterisitcs depending on the insulin receptor isoform involved. J. Biol. Chem. 277, 39684–39695 (2002).
Sakai, K. & Clemmons, D. R. Glucosamine induces resistance to insulin-like growth factor I (IGF-I) and insulin in Hep G2 cell cultures: biological significance of IGF-1/insulin hybrid receptors. Endocrinology 144, 2388–2395 (2003).
Rowinsky, E. K. et al. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin. Cancer Res. 13, 5549–5555 (2007).
Ulanet, D. B., Ludwig, D. L., Kahn, C. R. & Hanahan, D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic reistance to IGF-1R targeted therapy. Proc. Natl. Acad. Sci. USA 107, 10791–10798 (2010).
McKern, N. M. et al. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature 443, 218–221 (2006).
Bäck, K., Islam, R., Johansson, G. S., Chisalita, S. & Arnqvist, H. J. Insulin and IGF1 receptors in human cardiac microvascular endothelial cells: metabolic, mitogenic and anti-inflammatory effects. J. Endocrinol. 215, 89–96 (2012).
Soos, M. A. et al. A panel of monoclonal antibodies for the type I insulin-like growth factor receptor. J. Biol. Chem. 267, 12955–12963 (1992).
Soos, M. A. et al. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem. J. 235, 199–208 (1986).
Acknowledgements
Lene Petersen, Henrik Olsen, Marianne Fynbo Stendal and Dorte Nørgaard-Pedersen are thanked for excellent technical assistance.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Dr Slaaby is an employee at Novo Nordisk and owns stocks/shares in the company.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Slaaby, R. Specific insulin/IGF1 hybrid receptor activation assay reveals IGF1 as a more potent ligand than insulin. Sci Rep 5, 7911 (2015). https://doi.org/10.1038/srep07911
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07911
- Springer Nature Limited
This article is cited by
-
Insulin and IGF-1 have both overlapping and distinct effects on CD4+ T cell mitochondria, metabolism, and function
Scientific Reports (2024)
-
Hyperinsulinaemia in cancer
Nature Reviews Cancer (2020)
-
Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice
Nature Communications (2018)
-
Insulin mediates de novo nuclear accumulation of the IGF-1/insulin Hybrid Receptor in corneal epithelial cells
Scientific Reports (2018)
-
Biotin-transfer from a trifunctional crosslinker for identification of cell surface receptors of soluble protein ligands
Scientific Reports (2017)