Abstract
Solenopsis invicta and Solenopsis richteri are two closely related invasive ants native to South America. Despite their similarity in biology and behavior, S. invicta is a more successful invasive species. Toxic tolerance has been found to be important to the success of some invasive species. Esterases play a crucial role in toxic tolerance of insects. Hence, we hypothesized that the more invasive S. invicta would have a higher esterase activity than S. richteri. Esterase activities were measured for workers and male and female alates of both ant species using α-naphthyl acetate and β-naphthyl acetate as substrates. Esterase activities in S. invicta were always significantly higher than those in S. richteri supporting our hypothesis. In S. invicta, male alates had the highest esterase activities followed by workers then female alates for both substrates. In S. richetri, for α-naphthyl acetate, male alates had the highest activity followed by female alates then workers, while for β-naphthyl acetate, female alates had the highest activity followed by male alates then workers. For workers, S. richteri showed significantly higher levels of variation about the mean esterase activity than S. invicta. However, S. invicta showed significantly higher levels of variation in both female and male alates.
Similar content being viewed by others
Introduction
The red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae), is an infamous global invasive pest ant. Native to South America, S. invicta has been introduced into many countries and regions1 and has a great potential for further spread2. Solenopsis invicta is not only a significant threat to public health due to their venomous stings, but is also an important pest in agriculture by damaging crops and interfering with farming operations. Solenopsis invicta causes $6 billion in estimated annual economic loss in the United States alone3. The ecological impact of S. invicta is also enormous. For instance, S. invicta not only reduces species density of native ants at local scales, but also changes the co-occurrence patterns of surviving ant species at a biogeographic scale4. Due to its great social, economic and ecologic importance, S. invicta has been included in the “100 of the World's Worst Invasive Alien Species”5.
The black imported fire ant, Solenopsis richteri, also native to South America, is a species closely related to S. invicta. These two species invaded the United States through the same entrance: the port of Mobil, Alabama. Although S. richteri was introduced and established earlier than S. invicta6, the latter has successfully displaced S. richteri throughout most of its distribution6,7,8,9,10. In contrast to the wide global distribution of S. invicta, S. richteri has established outside its native range only in the southern United States and currently limited to small area in north Mississippi, north Alabama and southern Tennessee. These two species are very similar in morphology, biology, colony structure, foraging and feeding behavior and diet11, which caused confusion in the early literature as to species identity. Hybridization between these two species occurs in the United States12, indicating their high genetic and ecological similarity. However, the difference in the overall invasion success and their interaction in the United States indicate that S. invicta must possess some traits that provide a competitive advantage over S. richteri. S. invicta is a well-known disturbance specialist that thrives in the disturbed habitats6,13. It has been suggested that a species with higher toxic tolerance is likely to have a strong advantage in disturbed or polluted habitats14. Esterases plays a crucial role in toxic tolerance of insects due to their detoxification functions. A wide range of different esterases exist in insects and they differ in their substrate specificity and function15. Esterase isozymes in insects, particularly those in Drosophila and mosquitoes, are classified according to their preferential hydrolysis of the isomeric artificial substrates, α-naphthyl acetate (α-NA) and β-naphthyl acetate (β-NA)15. Although the classification has little value as a predictor of enzyme function, esterase activities measured using those substrates have often been proven to be associated with insecticide tolerance and resistance16,17,18,19,20,21,22,23,24,25,26. In this study, we tested a hypothesis that S. invicta has higher esterase activity than S. richteri. The esterase activities in workers and male and female alates of both species were analyzed using α-NA and β-NA. The kinetics of esterase was established for both species and substrates. The inhibition of esterase was also investigated using eserine, a carbamate, malaoxon, an organophosphate and 2-tridecanone, a common ant defensive compound.
Results
Esterase activity
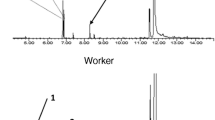
Esterase activities determined using enzyme preparation of 5 workers are shown in Fig. 1. Solenopsis invicta always had significantly higher esterase activity than S. richteri for both α-NA (t = 13.39, df = 67, P = 0.0002) and β-NA (z = −6.85, P < 0.0001). For each substrate, significant difference in esterase activity was found among colonies within each species except β-NA in S. invicta (α-NA in S. invicta: F2, 30 = 5.51, P = 0.0092; β-NA in S. invicta: H = 3.00, df = 2, P = 0.22; α-NA in S. richteri: F2, 33 = 8.64, P = 0.001; β-NA in S. richteri: F2, 33 = 10.10, P = 0.004).
Mean esterase activities (±SE) in workers of Solenopsis invicta and S. richteri for hydrolyzing α-naphthyl acetate (α-NA) and β-naphthyl acetate (β-NA), which were determined by using enzyme preparations of 5 workers.
The difference between two ant species is significant for both α-NA (t = 13.39, df = 67, P = 0.0002) and β-NA (z = -6.85, P < 0.0001).
Esterase activities determined by using individual ants are summarized in Table 1. Solenopsis invicta always had significantly higher mean esterase activity than S. richteri for both α-NA (worker: z = −11.26, P < 0.0001; female alate: z = −4.91, P < 0.0001; male alate: z = 9.07, P < 0.0001) and β-NA (worker: z = −13.29, P < 0.0001; female alate: z = −4.30, P < 0.0001; male alate: z = 11.11, P < 0.0001). Pairwise comparison between castes within a species was all significant for both substrates (P ≤ 0.0009) (Table 1S). Male alates had significantly higher esterase activity than workers for both species on both substrates. On α-NA, male alates had significantly higher esterase activity than female alates for both species. So did S. invicta male alates on β–NA. However, S. richteri female alates had higher activity than male alates on β–NA. Esterase activity in individual workers is shown in Fig. 2. For both substrates, S. richteri had a greater variance than S. invicta. The data for S. invicta was normally distributed for both substrates. In contrast, data for S. richteri were not normal for both substrates (Figure S1). Esterase activities of individual female alates are shown in Fig. 3. S. invicta had a greater variance than S. richteri for both substrates. The data for S. richteri was normally distributed, but not the data for S. invicta (Figure S2). Esterase activity in male alates is shown in Fig. 4. S. invicta also had a greater variance than S. richteri for both substrates. All data were not normally distributed except the data for S. richteri on β-NA (Figure S3).
Esterase kinetics
Kinetic parameters of esterases for α-NA and β-NA are shown in Table 2 and Table 3 respectively. Two types of Vmax values were calculated: one based on individual ants (μmol/min/ant) and the other on amount of protein (μmol/min/mg protein). Ratios of Km and Vmax values between two ant species (S. invicta/S. richteri) are shown in Table 4. For α-NA, the difference in Km values between two ant species was not significant (t = −0.79, df = 16, P = 0.44). The difference was significant among colonies in S. invicta (F2, 6 = 7.09, P = 0.03), but not in S. richteri (F2, 6 = 1.97, P = 0.22). Solenopsis invicta had significantly greater ant based Vmax (μmol/min/ant) than S. richteri (t = −5.33, df = 16, P < 0.0001). The difference among colonies was significant for both S. invicta (F2, 6 = 5.18, P = 0.049) and S. invicta (F2, 6 = 7.13, P = 0.026). Solenopsis invicta had greater protein based Vmax (μmol/min/mg protein) than S. richteri and the difference was very close to be statistically significant (t = −2.08, df = 16, P = 0.054). The difference among colonies was significant in S. invicta (F2, 6 = 10.84, P = 0.01) but not in S. richteri (F2, 6 = 3.12, P = 0.12). For β-NA, the difference in mean Km values was not significant between two ant species (t = −1.26, df = 16, P = 0.23). The difference was significant among colonies in S. invicta (F2, 6 = 6.73, P = 0.03), but not in S. richteri (F2, 5 = 5.54, P = 0.054). Solenopsis invicta had significantly greater ant based Vmax (μmol/min/ant) than S. richteri (t = −4.50, df = 15, P = 0.0004). The difference among colonies was not significant for both S. richteri (F2, 5 = 1.80, P = 0.25) and S. invicta (F2, 6 = 3.02, P = 0.12). Solenopsis invicta had greater protein based Vmax (μmol/min/mg protein) than S. richteri (t = −2.20, df = 15, P = 0.044). The difference among colonies was significant in S. invicta (F2, 6 = 7.97, P = 0.02) but not in S. richteri (F2, 5 = 2.95, P = 0.14).
Inhibition of esterase by 2-tridecanone, eserine and malaoxon
All three inhibitors caused a significant reduction in esterase activities in both species. Inhibition rates by 2-tridecanone and eserine are shown in Fig. 5. I50 values in inhibition by malaoxon are shown in Table 5. For α-NA, 2-tridecanone inhibited 6.74% and 25.90% esterase activity in S. invicta and S. richteri respectively. The difference between species was significant (t = 13.39, df = 67, P < 0.0001). The difference among colonies was significant for S. richteri (F2, 33 = 12.09, P = 0.0001), but not for S. invicta (F2, 30 = 0.02, P = 0.98). For β-NA, 2-tridecanone inhibited 7.34% and 15.66% esterase activity in S. invicta and S. richteri respectively. The difference was also significant between species (t = 5.88, df = 67, P < 0.0001). The difference among colonies was significant for S. richteri (F2, 30 = 3.44, P = 0.044) but not for S. invicta (F2, 30 = 2.44, P = 0.10).
For α-NA, eserine inhibited 63.86% and 60.40% esterase activity in S. invicta and S. richteri respectively (Fig. 5). The difference was significant between species (t = −3.73, df = 67, P = 0.0004). The difference among colonies was significant for both S. invicta (F2, 30 = 5.51, P = 0.0092) and S. richteri (F2, 33 = 8.64, P = 0.001). For β-NA, eserine inhibited 40.99% and 55.92% esterase activity in S. invicta and S. richteri respectively. The difference was also significant between species (t = 15.94, df = 67, P < 0.0001). The difference among colonies was significant for S. richteri (F2, 33 = 10.10, P = 0.0004), but not for S. invicta (F2, 30 = 2.58, P = 0.092). For S. invicta, the difference in inhibition rate by 2-tridecanone between two substrates was not significant (t = 0.45, df = 64, P = 0.65), but the difference was significant for eserine (t = 24.15, df = 64, P < 0.0001). For S. richteri, the difference in inhibition rate between two substrates was significant for both 2-tridecanone (t = 6.78, df = 70, P < 0.0001) and eserine (t = 4.88, df = 70, P < 0.0001).
Malaoxon inhibited esterase activity in both S. invicta and and S. richteri. For α-NA, malaoxon had greater I50 value for S. richteri than S. invicta (z = 3.378, P = 0.0004) and no significant difference was found among colonies for both species (S. invicta: H = 2.90, df = 2, P = 0.240; S. richteri: F2, 9 = 0.99, P = 0.41). For β-NA, the difference in I50 value was neither significant between two ant species (z = −0.032, P = 0.38), nor among colonies (S. invicta: H = 5.77, df = 2, P = 0.056; S. richteri: F2, 9 = 1.66, P = 0.24).
Discussion
This study clearly demonstrates that S. invicta has significantly higher esterase activity than S. richteri. As a critical component in insect's detoxification process, esterase has been intensively investigated in the context of insecticide resistance. Elevated esterase activities are associated with decreased insecticide susceptibility in numerous insects16,17,18,19,20,21,22,23,24,25,26. The detoxifying enzymes are important not only in tolerance and resistance of insects to both synthetic and natural toxins, but also in defense against microbial infection. For example, mosquito larvae with higher detoxification capability were more tolerant to toxicants from leaf litter27 and to infection caused by an entomopathogenic bacteria, Bacillus thuringiensis var. israelensis28. Esterases in Locusta migratoria were involved in the defense against the infection of an entomopathogenic fungus Metarhizium anisopliae29.
Having a high detoxification enzyme activity must be important to the success of ants, since they frequently encounter toxins and pathogenic microorganisms in their life. The most important natural enemies of ants are probably other ants. Many ants possess toxic venom which is utilized not only in their predation but also in their combats with other ants. In a combat, the venom is either injected, sprayed, or smeared to their enemies. Ants with higher tolerance to toxic venom may have an advantage in their competition with other ants. Many ants construct a nest that provides an ideal environment for pathogenic bacteria, fungi and other microbes to thrive. Adapting to the infection risks from those pathogenic microorganisms is believed to be one of driving forces in the evolution of the remarkable diversification of the social insects30. Ants with higher ability to defense against pathogenic microorganisms will definitely be more likely to survive in an unfavorable environment. The detoxification enzyme activity may be among the reasons why S. invicta is so successful.
2-Tridecanone is a common ant defensive chemical, which is used by many ant species, such as Paratrechina longicornis, Gigantiops destructor and 14 species within genus Myrmecocystus31,32,33. It has also been found in tawny crazy ant, Nylanderia fulva34. Tawny crazy ant is the only ant that is found in the field to be able to displace S. invicta35. Naturally occurring 2-tridecanone was first identified in the wild tomato Lycopersicon hirsutum f. glabratum36 and its insecticidal property has been demonstrated against insect and mite37,38. It is also a tick repellant39. 2-tridecanone may be a common natural toxin that both S. invicta and S. richteri often encounter. This study shows that esterase in S. invicta is significantly less sensitive to 2-tridecanone than S. richteri, indicating S. invicta may be less impacted by opponent's defense than S. richteri in competition with other ants, at least those ant species that utilize 2-tridecanone as their defensive chemical.
Based on their sensitivities to organophosphates (Ops), esterases are classified into three types: A-, B- and C-esterase40,41. Only B-esterases are readily inhibited by Ops. Esterases from both S. invicta and S. richteri are inhibited by malaoxon, indicating that they are most likely B-esterase. B-esterases can be further classified into carboxyesterases and cholinesterases based on their response to eserine15,42. Since esterases from both ant species are sensitive to eserine inhibition, cholinesterases and/or cholinesterase-like esterases may also be an important composition of esterases in both species.
Since male alates have limited exposure to the environment, it was unexpected to find that male alates in both species had the highest esterase activities when α-naphthyl acetate was used as a substrate. It indicates some unknown biological events specific to male alates require high esterase activity. In addition to detoxification, esterases play an important role in many other physiological activities in insects, such as the response to pheromone and plant volatiles43,44,45. What are the unique functions of esterases in male alates is an attractive subject for future research and it will also be interesting to see whether ants of different lineages have a similar trend.
In addition to detoxification enzymes, many other biological and/or ecological traits can contribute to the invasion success of a particular species. In plants, it has proven difficult to identify traits that consistently predict invasiveness and this may be largely because different traits favor invasiveness in different habitats46. This is most likely also true to invasive insects. Whether the difference in esterase activity observed in this study is indeed associated with the variation in invasion success between S. invicta and S. richteri can only be confirmed by further comparative studies between these two species and function analysis of such differences in different habitats.
Methods
Ants
Twenty Solenopsis invicta colonies were collected from Washington County, Mississippi and 20 S. richteri colonies from Desoto County, Mississippi. Permission for collecting ant colonies on highway right-of-way was issued by Mississippi Department of Transportation. Ant colonies were maintained in an insect rearing room at 25°C. All colonies used in this study were ensured to be free of Kneallhazia solenopsae, a microsporidian pathogen. The social form of S. invicta colonies was determined using PCR on Gp-9 alleles. Method described by Valles and Porter47 was used to amplify Gp-9 alleles. All ants used in this study were from monogyne colonies. Mature queen-right colonies with brood and alates were used in the study. All colonies were on the same diet (10% sugar water and house crickets). Only newly collected ant colonies were used.
Chemicals
2-tridecanone, Fast Blue B salt [o-Dianisidine bis(diazotized) zinc double salt; DBB], sodium dodecyl sulfate (SDS), α-naphthyl acetate (α-NA), β-naphthyl acetate (β-NA) α-naphthol, β-naphthol, malaoxon and eserine were purchased from Sigma Aldrich (St Louis, MO, USA). Stock solutions of both α-NA and β-NA were prepared in absolute ethanol.
Enzyme preparation
In addition to individual ants, the esterase activity was also determined using enzyme preparation of 5 workers. The enzyme preparations of 5 workers were also used in determining esterase kinetics and inhibition. Ant(s) was grinded using a pestle in a 1.5-ml centrifuge tube after being frozen at −80°C for 24 h. The sample was homogenized thoroughly for about 1 min at 4,000 rpm in 40 mM sodium phosphate buffer (pH 7.4) containing 0.02% Triton X-100. Five hundred microliter of sodium phosphate buffer was used for the preparation of 5 workers; 200 μl for individual worker; 400 μl for individual alate (male or female). The preparation was then centrifuged at 8,000 rpm for 10 min by using a microcentrifuge (Beckman Coulter, Fullerton, CA). The supernatant was decanted and filtered through glass wool. Before enzyme bioassay, for the preparation of 5 workers, the supernatant was diluted 10 fold for S. invicta and 5 fold for S. richteri. No dilution was made for preparations of individual ant.
Esterase activity
A colorimetric method was adapted for measuring esterase activity using α-NA or β-NA as substrates48. In brief, esterases hydrolyzed α- or β-naphthyl acetate into α- or β-naphthol and acetate. The amount of α- or β-naphthol produced was used to determine the esterase activity. A reaction mixture consisted of one of the following preparations in a 1.5-ml centrifuge tube:
-
1
40 μl of enzyme preparation of 5 workers (0.04 and 0.08 ant equivalent for S. invicta and S. richteri respectively), 950 μl 40 mM sodium phosphate buffer (pH 7.4) and 10 μl 1 × 10−2 M α-NA or 5 × 10−3 M β-NA.
-
2
10 μl of individual female alate preparation, 980 μl 40 mM sodium phosphate buffer (pH 7.4) and 10 μl 2.5 × 10−3 M α-NA or β-NA.
-
3
20 μl of individual worker or male alate preparation, 970 μl 40 mM sodium phosphate buffer (pH 7.4) and 10 μl 2.5 × 10−3 M α-NA or β-NA.
The final volume of the mixture was 1.0 ml. After the mixture was incubated at 37°C for 30 minutes, 150 μl Fast Blue B-SDA–SDS solution (1.0% Fast Blue salt: 5% SDS = 2:5) was added to stop the reaction. Color was developed for 10 min and the absorbance was then measured using a UV-VIS spectrophotometer (Spectro UV-Vis Auto UV-2602, Labomed Culver City, CA) at λ = 600 nm for α-naphthol or λ = 560 nm for β-naphthol. The blank cuvette contained all ingredients except substrate. The concentration of α- or β-naphthol was determined from a standard curve of α- or β-naphthol. For determining esterase activities using preparations of 5 workers, 3 colonies were used for each species and there were 9–12 replicates for each colony. For individual worker, 7 colonies were used for each species and there were 20 replicates for each colony. For individual female alate, 4 S. richteri colonies and 5 S. invicta colonies were used. There were 12 to 24 replicates for each colony. For individual male alate, 8 S. richteri colonies and 4 S. invicta colonies were used. There were 15 to 35 replicates for each colony.
Determination of protein contents
Protein content in the enzyme preparation of 5 workers was determined for each enzyme preparation using the method described by Bradford49. Bovine serum albumin was used as the standard and absorbance was measured at λ = 595 nm. Protein content in the enzyme preparation of individual ant was not determined.
Esterase kinetics
Vmax, the enzyme's maximum velocity and Km, the Michaelis constant, were determined for both α-NA and β-NA using enzyme preparations of 5 workers. Reaction mixture consisted of 50 μl diluted enzyme preparation, 940 μl 0.04 M sodium phosphate buffer (pH 7.4) and one of the following six concentrations of substrate: 2.5 × 10−3, 5 × 10−3, 2.5 × 10−2, 5 × 10−2, 2.5 × 10−1 and 5 × 10−1 mM. Color development and absorbance measurement were the same as described above. Vmax and Km, were calculated using Lineweaver-Burk Plot50. Three colonies were used for each species. There were 2 to 4 replicates for each colony.
Inhibition of esterase by 2-tridecanone, eserine and malaoxon
The inhibition of esterase activity by 2-tridecanone, esterine and malaoxon was measured for both α- and β-naphthyl acetate using enzyme preparations of 5 workers. 2-tridecanone and eserine were tested only at 0.25 M and 5 × 10−4 M respectively; whereas 5 concentrations of malaoxon were tested, including 1 × 10−3, 5 × 10−4, 1 × 10−4, 5 × 10−5 and 1 × 10−5 M. Test compound (5 μl) was added in a 1.5 ml centrifuge tube. In control, only acetone, the solvent for all tested compounds, was added. After acetone was completely evaporated (10 min) at room temperature, 40 μl diluted enzyme preparation was added into the tube. After the mixture was pre-incubated at 37°C for 10 min, 945 μl 40 mM sodium phosphate buffer (pH 7.4) and 10 μl 2.5 × 10−3 M α-NA or β-NA were added. After the mixture was incubated at 37°C for 30 minutes, 150 μl Fast Blue B-SDA–SDS solution was added to stop the reaction. The inhibition rates (%) for 2-tridecanone and eserine and I50 values (concentration that causes 50% enzyme inhibition) for malaoxon were calculated. Three colonies from each species were used for each compound. There were 9–12 replicates from each colony for inhibition rate of 2-tridecanone and serine and 3–5 replicates for I50 value of malaoxon.
Data analysis
Whenever data was normally distributed, a t-test was used for pairwise comparison in difference of esterase activity, parameter of enzyme kinetics, inhibition rate and I50 value between two ant species using pooled cross-colony data. Otherwise, Mann–Whitney U test was used. For comparison among colonies, analysis of variance (PROC GLM, SAS Institute 2008) was used when data was normally distributed, otherwise Kruskal-Wallis test was used.
References
Ascunce, M. S. et al. Global invasion history of the fire ant Solenopsis invicta. Science 331, 1066–1068 (2011).
Morrison, L. W., Porter, S. D., Daniels, E. & Korzukhin, M. D. Potential global range expansion of the invasive fire ant. Solenopsis invicta. Biol. Invasions 6, 183–191 (2004).
Lard, C. F. et al. An economic impact of imported fire ants in the United States of America. Texas A & M University, College Station, Texas, USA (2006) Available at: (http://www.extension.org/sites/default/files/Copy%20of%20the%20National%20Study.pdf) (Accessed: 2nd October 2014).
Gotelli, N. J. & Arnett, A. E. Biogeographic effects of red fire ant invasion. Ecol. Lett. 3, 257–261 (2000).
Lowe, S., Browne, M., Boudjelas, S. & De Poorter, M. 100 of the World's Worst Invasive Alien Species: A selection from the Global Invasive Species Database Published by The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN) p.1–12 (2000).
Tschinkel, W. R. The fire ants. (Harvard University Press, 2006).
Callcott, A. M. Range expansion of the imported fire ant-1918–2001 (updated). (p. 91–93, In: D. Suiter, et al., [compiliers], Proceedings of the 2002 Imported Fire Ant Conference, 24–26 March 2002, Athens, GA, 173 p., 2002).
Oliver, J. B. et al. Statewide survey of imported fire ant (Hymenoptera: Formicidae) populations in Tennessee. J. Entomol. Sci. 44, 149–157 (2009).
Diffie, S., Vander Meer, R. K. & Bass, M. H. Discovery of hybrid fire ant populations in Georgia and Alabama. J. Entomol. Sci. 23, 187–191 (1988).
Streett, D. A., Freeland, T. B., Jr & Vander Meer, R. K. Survey of imported fire ant (Hymenoptera: Formicidae) populations in Mississippi. Fla. Entomol. 89, 91–92 (2006).
Taber, S. W. Fire ants. (Texas A&M University Press, 2000).
Vander Meer, R. K., Lofgren, C. S. & Alvarez, F. M. Biochemical evidence for hybridization in fire ants. Fla. Entomol. 68, 501–506 (1985).
King, J. R. & Tschinkel, W. R. Experimental evidence that human impacts drive fire ant invasions and ecological change. Proc. Natl. Acad. Sci. U. S. A. 105, 20339–20343 (2008).
Weir, S. M. & Salice, C. J. High tolerance to abiotic stressors and invasion success of the slow growing freshwater snail, Melanoides tuberculatus. Biol. Invasions 14, 385–394 (2012).
Oakeshott, J. G., Claudianos, C., Campbell, P. M., Newcomb, R. D. & Russell, R. J. Biochemical genetics and genomics of insect esterases. in Comprehensive molecular insect science (ed Kostas Iatrou and Sarjeet S. Gill Lawrence I. Gilbert) 309–381 (The Netherlands: Elsevier, 2005).
Gordon, J. R. & Ottea, J. Association of esterases with insecticide resistance in Culex quinquefasciatus (Diptera: Culicidae). J. Econ. Entomol. 105, 971–8 (2012).
Low, V. L. et al. Enzymatic Characterization of insecticide resistance mechanisms in field populations of Malaysian Culex quinquefasciatus Say (Diptera: Culicidae). PLoS ONE 8, e79928 (2013).
Marcombe, S., Farajollahi, A., Healy, S. P., Clark, G. G. & Fonseca, D. M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 9, e101992 (2014).
Teese, M. G. et al. Heterologous expression and biochemical characterisation of fourteen esterases from Helicoverpa armigera. PLoS ONE 8, e65951 (2013).
Zhu, K. Y. & Brindley, W. A. Significance of carboxylesterases and insensitive acetylcholinesterase in conferring organophosphate resistance in Lygus hesperus populations. Pest. Biochem. Physiol. 43, 223–231 (1992).
Bisset, J. A. et al. Insecticide resistance in two Aedes aegypti (Diptera: Culicidae) strains from Costa Rica. J. Med. Entomol. 50, 352–361 (2013).
Smirle, M. J., Zurowski, C. L., Lowery, D. T. & Foottit, R. G. Relationship of insecticide tolerance to esterase enzyme activity in Aphis pomi and Aphis spiraecola (Hemiptera: Aphididae). J. Econ. Entomol. 103, 374–378 (2010).
Zhu, Y. C., Guo, Z., He, Y. & Luttrell, R. Microarray analysis of gene regulations and potential association with acephate-resistance and fitness cost in Lygus lineolaris. PLoS ONE 7, e37586 (2012).
Zhu, Y. C., Snodgrass, G. L. & Chen, M. S. Enhanced esterase gene expression and activity in a malathion-resistant strain of the tarnished plant bug, Lygus lineolaris. Insect Biochem. Mol. Biol. 34, 1175–1186 (2004).
Jackson, C. J. et al. Structure and function of an insect α-carboxylesterase (αEsterase7) associated with insecticide resistance. Proc. Natl. Acad. Sci. U S A 110, 10177–10182 (2013).
Zhu, K. Y. & Brindley, W. A. Properties of esterases from Lygus hesperus (Hemiptera: Miridae) and the roles of the esterases in insecticide resistance. J. Econ. Entomol. 83, 725–732 (1990).
Tilquin, M., Meyran, J. C. & Marigo, G. Comparative capability to detoxify vegetable allelochemicals by larval mosquitoes. J. Chem. Ecol. 30, 1381–1391 (2004).
Boyer, S., Tilquin, M. & Ravanel, P. Differential sensitivity to Bacillus thuringiensis var. israelensis and temephos in field mosquito populations of Ochlerotatus cataphylla (Diptera: Culicidae): toward resistance? Environ. Toxicol. Chem. 26, 157–162 (2007).
Dubovskiy, I. M. et al. The activity of nonspecific esterases and glutathione-S-transferase in Locusta migratoria larvae infected with the fungus Metarhizium anisopliae (Ascomycota, Hypocreales). Entomol. Rev. 92, 27–31 (2012).
Hölldobler, B. & Wilson, E. O. The ants. (Harvard University Press, Cambridge, 1990).
Morgan, E. D., Jackson, B. D. & Billen, J. Chemical secretions of the “crazy ant” Paratrechina longicornis (Hymenoptera: Formicidae). Sociobiology 46, 299–304 (2005).
Blum, M. S. et al. Exocrine chemistry of the monotypic ant genus Gigantiops. Comp. Biochem. Physiol. B Comp. Biochem. 75, 15–16 (1983).
Lloyd, H. A., Blum, M. S., Snelling, R. R. & Evans, S. L. Chemistry of mandibular and Dufour's gland secretions of ants in genus Myrmecocystus. J. Chem. Ecol. 15, 2589–2599 (1989).
Chen, J., Rashid, T., Feng, G., Oi, D. & Dreese, B. M. Defensive chemicals of tawny crazy ants, Nylanderia fulva (Hymenoptera: Formicidae) and their toxicity to red imported fire ants, Solenopsis invicta (Hymenoptera: Formicidae). Toxicon 76, 160–166 (2013).
LeBrun, E. G., Abbott, J. & Gilbert, L. E. Imported crazy ant displaces imported fire ant, reduces and homogenizes grassland ant and arthropod assemblages. Biol Invasions 15, 2429–2442 (2013).
Williams, W. G., Kennedy, G. G., Yamamoto, R. T., Thacker, J. D. & Bordner, J. 2-Tridecanone: a naturally occurring insecticide from the wild tomato Lycopersicon hirsutum f.glabratum. Science 207, 888–889 (1980).
Antonious, G. F. & Snyder, J. C. Natural products: repellency and toxicity of wild tomato leaf extracts to the two-spotted spider mite, Tetranychus urticae Koch. J. Environ. Sci. Health B 41, 43–55 (2006).
Braga, Y. F. et al. Insecticidal activity of 2-tridecanone against the cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae). An. Acad. Bras. Cienc. 79, 35–39 (2007).
Kimps, N. W., Bissinger, B. W., Apperson, C. S., Sonenshine, D. E. & Roe, R. M. First report of the repellency of 2-tridecanone against ticks. Med. Vet. Entomol. 25, 202–208 (2011).
Bergmann, F., Segal, R. & Rimon, S. A new type of esterase in hog-kidney extract. Biochem. J. 67, 481–486 (1957).
Aldridge, W. N. Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate and a method for their determination. Biochem. J. 53, 110–117 (1953).
He, Y.-p., Ma, E.-B. & Zhu, K.-Y. Characterizations of general esterases in relation to malathion susceptibility in two field populations of the oriental migratory locust, Locusta migratoria manilensis (Meyen). Pest. Biochem. Physiol. 78, 103–113 (2004).
He, P. et al. Functional characterization of an antennal esterase from the noctuid moth, Spodoptera exigua. Arch. Insect Biochem. Physiol. 86, 85–99 (2014).
Chertemps, T. et al. carboxylesterase, esterase-6, modulates sensory physiological and behavioral response dynamics to pheromone in Drosophila. BMC Biol. 10, 56 (2012).
Kontogiannatos, D. et al. Functional characterization of a juvenile hormone esterase related gene in the moth Sesamia nonagrioides through RNA interference. PLoS One 8, e73834 (2013).
Alpert, P., Bone, E. & Holzapfel, C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect. Plant. Ecol. Evol. Syst. 3, 52–66 (2000).
Valles, S. M. & Porter, S. D. Identification of polygyne and monogyne fire ant colonies (Solenopsis invicta) by multiplex PCR of Gp-9 alleles. Insectes Soc. 50, 199–200 (2003).
van Asperen, K. A study of housefly esterase by means of a sensitive colorimetric method. J. Insect Physiol. 8, 401–414 (1962).
Bradford, M. M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Lineweaver, H. & Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56, 658–666 (1934).
Acknowledgements
We thank Dr. Gregg Henderson, Department of Entomology, Louisiana State University, Baton Rouge, LA and Dr. Yucheng Zhu, USDA-ARS, Stoneville, MS for critical reviews of the manuscript. We thank Mr. Leon Hicks and Ms. Weihua Jiang for their technical assistance. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U. S. Department of Agriculture.
Author information
Authors and Affiliations
Contributions
Conceived the experiments: J.C. and T.R. Designed the experiment: J.C. and G.F. Performed the experiments: G.F. Analyzed the data: J.C., G.F. and T.R. Wrote the manuscript: J.C. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Chen, J., Rashid, T. & Feng, G. Esterase in Imported Fire Ants, Solenopsis invicta and S. richteri (Hymenoptera: Formicidae): Activity, Kinetics and Variation. Sci Rep 4, 7112 (2014). https://doi.org/10.1038/srep07112
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07112
- Springer Nature Limited