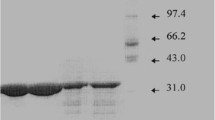
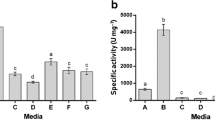
An alkaline protease produced by Pseudomonas aeruginosa MN1, isolated from an alkaline tannery waste water, was purified and characterized. The enzyme was purified 25-fold by gel filtration and ion exchange chromatography to a specific activity of 82350 U mg−1. The molecular weight of the enzyme was estimated to be 32000 daltons. The optimum pH and temperature for the proteolytic activity were pH 8.00 and 60°C, respectively. Enzyme activity was inhibited by EDTA suggesting that the preparation contains a metalloprotease. Enzyme activity was strongly inhibited by Zn2+, Cu2+ and Hg2+(5 mM), while Ca2+ and Mn2+ resulted in partial inhibition. The enzyme is different from other Pseudomonas aeruginosa alkaline proteases in its stability at high temperature; it retained more than 90% and 66% of the initial activity after 15 and 120 min incubation at 60°C. Journal of Industrial Microbiology & Biotechnology (2000) 24, 291–295.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 09 June 1999/ Accepted in revised form 24 January 2000
Rights and permissions
About this article
Cite this article
Bayoudh, A., Gharsallah, N., Chamkha, M. et al. Purification and characterization of an alkaline protease from Pseudomonas aeruginosa MN1. J Ind Microbiol Biotech 24, 291–295 (2000). https://doi.org/10.1038/sj.jim.2900822
Issue Date:
DOI: https://doi.org/10.1038/sj.jim.2900822