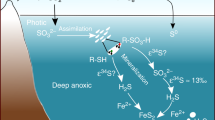
Abstract
Bulk-rock sulfur isotope data from pyrite in the ~2.1 billion-year sedimentary rocks of the Francevillian Basin, Gabon, have underpinned ideas about initial oxygenation of Earth’s surface environments and eukaryote evolution. Here, we show, using micro-scale analytical methods, that the bulk sulfur isotope record represents progressive diagenetic modification. Our findings indicate no significant change in microbial sulfur cycling processes and seawater sulfate composition throughout that initial phase of atmosphere-ocean oxygenation of Paleoproterozoic time. This offers an alternative view of Earth system evolution during the transition from an anoxic to an oxic state and highlights the need for a judicious reappraisal of conceptual models using sulfur isotope data as primary depositional signals linked to global-scale biogeochemical processes.
Similar content being viewed by others
Introduction
Coupled excursions of carbon (C) and sulfur (S) isotopes obtained from ancient sedimentary successions have underpinned ideas about the redox evolution of Earth’s surface1,2,3. A consensus view is that the transition to a permanently oxygenated surface environment began with the Great Oxidation Event (GOE)~2.5–2.2 billion-years (Ga) ago4,5. However, low levels of atmospheric oxygen and ocean oxidant pools may have persisted for a long time afterward4. Following the GOE, instability in the redox conditions of the atmosphere-ocean system is believed to have been imprinted in the sedimentary record as large-scale disturbances, such as the 2.3–2.0 Ga Lomagundi-Jatuli positive carbon isotope Excursion (LJE), which is the largest and longest such event in Earth’s history3,6,7,8.
Such ideas are founded on the assumption that minerals from which stable isotope data are obtained reliably archive information about environmental conditions and that secondary overprints during diagenesis and weathering are minimal. Here, we critically assess that assumption using C and S isotope data from a well-preserved sedimentary succession that has been central in ideas about early Paleoproterozoic Earth System evolution, the ~2.1 Ga Francevillian Group of Gabon9 (Fig. 1). The Francevillian strata are thought to record the zenith of the GOE, termination of the LJE, and a global deoxygenation episode (O2 ‘overshoot and collapse’)10 of the atmosphere-ocean system3,7,11. However, a recent re-evaluation of the relationship between facies and C-isotope profiles from LJE-bearing carbonate rocks worldwide has shown that the LJE excursion is a consequence of basin-specific conditions, not wholesale Earth system change12,13,14. This finding matches others that have likewise shown the variable dependency of paleoredox indicators (δ34S, δ13Corg, Fe-speciation, trace element, and redox-sensitive metal concentrations) on depositional facies, as well as their susceptibility to modification by diagenesis and weathering15,16,17,18. These insights motivated us to re-examine the S-isotope record of the Francevillian rocks.
A Simplified geological map and stratigraphy of the Paleoproterozoic Lastoursville sub-basin, Gabon20. White circles show the locations of drill cores and Lastoursville city. B Simplified map showing the location of Francevillian Group, Gabon. C Fresh outcrop in a quarry in the Lastoursville sub-basin where the LST12 core was drilled (0.8089°S, 12.7709°E). Pyrite nodules within a black shale bed are visibly oxidized, and various sulfate salts (white and yellow) precipitate along fractures. The yellow sulfate salt was determined to be copiapite (hydrated Fe-sulfate). The soluble sulfate salts easily dissolve during the rainy season and precipitate during the dry months.
Results
Geological setting and materials
The Paleoproterozoic Francevillian Group rests unconformably on Archean basement and outcrops in four intra-cratonic sub-basins: the Booué, Lastoursville, Okondja, and Franceville that together cover an area of ~44,000 km2 (Fig. 1)19. Five formations have been defined from the base upward: FA - sandstone and minor conglomerate; FB - sandstone, black shale, and dolostone; FC - dolostone, chert, and jasper; FD - mostly black shale; and FE - fine sandstone19. Sedimentation occurred in continental platform6 to margin settings19 and thicknesses vary from several tens to many hundreds and possibly several thousands of meters across the region20. The succession was mildly folded, cut by high-angle faults, and experienced low-grade metamorphism (up to 200–250 °C)21 during the 2 Ga Eburnean orogeny22. The FA–FC formations record a phase of basin opening, whereas FD and FE formations were deposited during the closing stages of the basin and subsequent erosion of the orogen22. The minimum depositional age for FA to the top of FB formations is 2191 ± 13 Ma, based on a U-Pb zircon age from the N’goutou Complex granite that intrudes both formations23. The age of FD Formation is constrained by U-Pb zircon ages of 2083 ± 6 Ma24, revised to 2003 ± 16 Ma22, from a tuff layer and 2072 ± 29 Ma on a detrital grain20.
Drill core record
Outcrops of the Francevillian rocks show signs of intense weathering (Fig. 1C); thus, we focused our studies on two drillcores: the 139 m long LST12 and 76 m long Doumé cores (Fig. 2). These are from the Lastoursville sub-basin and recover portions of the FB, FC, and FD formations deposited during the post-GOE through LJE time interval. The cores are within ~30 km of one another, and their litho- and chemo-stratigraphic similarities indicate that LST12 units IV–VI (formerly defined12) and Doumé units I–III (this study) partly overlap to define a shallow-marine shelf to deeper basin transect12.
Lithostratigraphic profiles of the LST12 and Doumé sections plotted alongside stable C and S isotope data, and total organic carbon (TOC), pyrite, dolomite, calcite, and manganese (Mn) content. Errors for the bulk and SIMS stable isotope data are encompassed within the data points. Core LST12 TOC, Mn, mineral, and carbon isotope compositions are from12.
Modern weathering affects the top 20 m in the LST12 core and top 8 m in the Doumé core. Additionally, the uppermost ~15 m of the Doumé core is strongly albitized, and we exclude those intervals from our discussion. Between the cores, a total of 64 samples below the visibly weathered intervals were selected for bulk sulfur isotope analysis, a subset of four samples for secondary ion mass spectrometry (SIMS), and ten for synchrotron-based analysis to investigate S speciation. We report pyrite petrography and S isotope and speciation data for the LST12 core, together with previously reported12 major and minor elements, mineral, and carbon isotope data. For the Doumé core, petrography, major and minor elements, and mineral and carbon stable isotope compositions are summarized below.
Bulk isotopic signatures and S species
In both cores, the trends of sulfur (δ34SCRS), organic (δ13Corg), and carbonate carbon (δ13Ccarb) values track one another in tandem with changes in composition: the isotopic values decrease with increasing concentrations of total organic carbon (TOC, from <1 wt.% up to 14.1 wt.%) and pyrite (from <3 wt.% up to 16.9 wt.%), and decreasing concentrations of dolomite, calcite, and Mn/Fe-rich carbonate from abundant to below a few wt.% in black shales (Supplementary Data 1, 2). The measured bulk chromium reducible sulfur (CRS) δ34SCRS reach values as high as 50.9‰ in carbonate beds (average 19.3 ± 12.0‰, 1σ here and elsewhere, n = 42), with negative values only found in a few black shale beds in LST12 units IV and VI and Doumé units I and III (min. −25.9‰, average 6.9 ± 13.5‰, n = 22). Measured organic sulfur compounds (OSC) have δ34SOSC values between −2.0 – 30.7‰ and are 34S enriched by <10‰ compared to paired δ34SCRS. Except for one outlier, organic sulfur δ34SOSC and pyrite δ34SCRS display a strong linear relationship (R2 = 0.9, p < 0.05; Fig. 3). Based on X-ray Absorption Near Edge Structure (XANES) spectroscopy, these compounds are a mixture of organic sulfur species dominated by aromatic and oxidized moieties, as indicated by XANES features at 2473.7 and 2480.8 eV, respectively (Fig. S1, Supplementary Data 3, 4).
Acid-extractable sulfate (δ34SAES) representing secondary sulfate was leached from the upper black shale units in both cores and ranges from −14.0 to 15.1‰. Bulk δ34SAES positively correlates with δ34SCRS (R2 = 0.7, p < 0.05) and is ~9‰ (± 3.4‰) lower than those of the associated bulk pyrite results (Figs. 2, 3B). The XANES spectra show a prominent peak at ~2482–2483 eV, indicating that inorganic sulfate salts such as Ca- and Fe-sulfates comprise the acid-extractable sulfate fraction (e.g., Figure. S1g–i). The inorganic sulfates are confined to cross-cutting veinlets, bed-parallel microfractures, and, in places, line pyrite aggregates (Fig. 4). All samples with inorganic sulfate spectra also display sulfide peaks (2471.8 eV) with differing intensities (e.g., Fig. S1h–l).
Tricolor S speciation maps of representative samples from the LST12 core from A black shale with Mn-rich carbonate at 31.1 m, B black shale at 53.8 m, and C grey dolostone with a quartz-pyrobitumen vein at 75.5 m depth using data from XANES fitting where red corresponds to inorganic sulfide, green to organic sulfur compounds (OSC), and blue to inorganic sulfate. The magenta on C shows regions where inorganic sulfide and sulfate co-occur; the bright blue and cyan lining and within the pyrobitumen vein, respectively, are secondary inorganic sulfate phases, and the dull diffused blue is carbonate-associated sulfate.
Pyrite formation and S isotope signatures
Pyrite in the studied samples is texturally variable and can be categorized into four types: type 1 disseminated minute crystals (typically octahedral <10 µm); type 2 octahedral pyrite (~10 to 20 µm) in stratiform laminae; type 3 aggregated pyrite composed of coarser octahedral or blocky cubic crystals (10s- to 100s-of-µm); and type 4 small and large cubic forms (10s- to 100s-of-µm) (Fig. 5). Common to all studied lithofacies in the LST12 and Doumé successions is that all pyrite types nucleated on a precursor phase (Fig. 5AIV, BIV, CV, DIV, DV). Whether those precursors were pyrite framboids, Fe-monosulfides, Fe-(hydr)oxides, or all of those is difficult to determine. This phase was subsequently engulfed by new pyrite growth that differed between black shales and carbonate rocks (Fig. 5 BIV, CIV, DIII–V, Fig. 6). In the latter, pyrite paragenesis shows a consistent pattern starting with a precursor that euhedral pyrite (type 1 or 4) nucleated on and were subsequently inter- or overgrown by larger cubes (type 4). In contrast, in black shales, type 1 pyrite transforms to type 2 pyrite in bedding-parallel organic-rich (now pyrobitumen) bands. In turn, these forms are encased by type 3 pyrite, forming matrix-supported (Fig. 5 BIII) or massive nodules (Fig. 5CIII). The type 2 pyrite and matrix-supported type 3 nodules display oscillatory backscatter intensity zonation in SEM BSE images (Fig. 5BIV, CIV). In places, the continued growth of nodules resulted in bending and fracturing of the stratiform bands and sediment layers (Fig. 5CIII). Alongside micro-fractures, the pyrite nodules and stratiform laminae have abundant small (<2 µm) discrete or interconnected rounded and polygonal pits that penetrate the crystals to varying depths and cut primary zonation (Fig. S2). The morphology of those pits resembles microbially induced or abiogenic dissolution patterns observed on pyrite from laboratory oxidation experiments25. These textures are uncommon in fine pyrite crystals disseminated within the black shale matrix and are lacking in the carbonate beds (Fig. 5AIV, BV, DIII–V).
Histograms of the SIMS δ34SPY data and scanning electron microscopy backscattered electron (SEM BSE) images of characteristic pyrite textures for samples A DM-191, B LST12-31.3, C LST12-53.8, and D LST12-79. The dark grey dashed and solid lines on the histograms represent the SIMS average and weighted average δ34SPY* value, respectively. The bin sizes (5‰) are larger than typical errors associated with spot and ion imaging results. Violin plots are used to show the distribution of δ34SPY for different pyrite textures. The black circles diameter is 2.5‰ in the violin plots. The black dashed lines in panels b3 and c3 mark nodule boundaries used for ImageJ particle analysis.
Pyrite formation in A black shale and B dolostone from early- to late-diagenesis and secondary sulfate mineralization. On the right C, Rayleigh models of residual sulfate (solid grey line) and MSR produced sulfide (black lines) δ34S in sediment pore waters assuming a starting sulfate of 10‰ (dashed grey line) and ε value of 45‰. The fraction of sulfate that had to be consumed to reach the measured SIMS δ34SPY is shown as colored circles. Notice that the accumulative sulfide’s (dashed black line) isotopic evolution fails to explain the highest measured δ34SPY values.
Mapping by laser-ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) shows distinct distribution patterns in type 2 and 3 pyrite for trace elements (Mn, Co, Ni, Cu, As, Mo, and Sb; Figs. S3–S6). Most notably, individual type 2 pyrite and its encased remnants in massive stratiform and nodule pyrite exhibit oscillatory core-to-rim zonation of Co, Ni, Cu, and Sb and lower Mn, As, and Mo concentrations relative to the overgrowing pyrite (Fig. S3–S6). Type 4 pyrite is the least abundant texture in the calcareous and black shales and templates on earlier pyrite or in a few cases replaces other mineral phases. Based on these textural relationships and chemical compositions, pyrite paragenesis in black shales involved the initial crystallization of type 1 on a precursor phase, followed by successive and overlapping growth of types 2, 3, and then type 4 pyrite. Some type 1 pyrite was trapped as inclusions within paragenetically later carbonate phases (i.e., Fig. 5BV). Lastly, pyrite was subjected to abiotic or biotic oxidative dissolution alongside micro-fractures (Fig. S2).
Secondary ion mass spectrometry measurements of four samples representative of different facies (31.3, 53.8, and 79.0 m depths in core LST12; 191 m depth in core Doumé, Supplementary Data 5) show that type 1 pyrite has the broadest δ34SPY range (mean δ34SPY 0.8 ± 17.4‰, n = 224, range ~87‰) and type 2 is the most 34S-depleted (mean δ34SPY −5.5 ± 10.3, n = 143, range ~53‰; Fig. 5AII, BII). Type 3 has low δ34SPY values in matrix-supported nodules (−12.6 ± 8.0‰, n = 78 in LST12–31.3; Fig. 5BII) and high δ34SPY values in massive nodules (20.4 ± 3.8‰, n = 34 in LST12-53.8; Fig. 5CII). Pyrite type 4 (mean δ34SPY 11.8 ± 8.9‰, n = 37, range ~35‰) and massive type 3 nodules (range ~13‰) are the most 34S-enriched and display the least δ34SPY variability (Table 1). Notably, the remnants of earlier pyrite within the massive nodules preserve distinct S isotope and trace element signature (Figure. S3 b2). When accounting for the relative surficial abundances of the different pyrite textures determined from SEM BSE images using ImageJ particle analysis26, weighted SIMS mean (δ34SPY*) values are within 3‰ of bulk δ34SCRS for all samples (LST12–79.0: 10.7‰ δ34SPY* and 13.9‰ δ34SCRS, LST12-53.8: 15.1‰ δ34SPY* and 18.0‰ δ34SCRS, LST12-31.3: −7.2‰ δ34SPY* and −10.2‰ δ34SCRS, DM-191: 3.8‰ δ34SPY* and 0.7‰ δ34SCRS; Fig. 5, Table 1). The consistency between the bulk and in situ results provides confidence that our SIMS data are representative of the analyzed samples and provide important insights about isotopic contrast between pyrite populations.
Discussion
The documentation of broadly similar chemostratigraphic trends in several Paleoproterozoic sedimentary basins has been used to argue that, following the GOE, oscillatory ocean redox conditions and widespread euxinia occurred3,6,7,8. Our complementary sedimentological, petrographic, and multi-proxy chemostratigraphic dataset challenges this idea by highlighting the effects of strong local forcing on C and S isotope records. The δ13C and δ34S profiles from the LST12 and Doumé cores are synchronous with stratigraphic variations that are linked directly to facies – values increase in carbonate-dominated strata and decrease in black shale. Further, the geochemical records in the FB – FD rocks, particularly those sampled in outcrops and shallow drillcores, appear more pervasively influenced by post-depositional oxidative alteration than appreciated by previous work7,8,11,27,28,29. In many of those earlier studies, the Francevillian rocks were thought to be unmetamorphosed and exceptionally well preserved. Instead, our integration of bulk and high resolution (wet-chemical extractions, petrography, SIMS, synchrotron X-ray, LA-ICP-MS) techniques show that hydrofracturing and oxidative alteration affected the geochemical signals of the Francevillian rocks far more than appreciated previoulsy, confirming the findings of other studies30,31,32,33. One such example is pyrite nodules sampled from a quarry in the Franceville sub-basin28,29 (FB formation), interpreted by some workers as well preserved and thus suitable for paleo-redox investigations11,27. However, these nodules have been oxidized as evidenced by iron-oxide rinds and XRD analysis showing the presence of goethite (Fe-oxyhydroxide)- a typical pyrite oxidation product- at the edges and centers of the nodules29. This highlights the necessity for undertaking evaluation of the influence of secondary alteration even from what are thought to be pristine outcrop and near-surface core samples, particularly in regions of intense tropical weathering that can extend to tens of meters into the subsurface34.
The inorganic sulfide (2471.8 eV) and sulfate features (~2482 eV) in the XANES spectra from the LST12 and Doumé samples, even at >70 m core depths, and the broadly positive δ34S relationship between the two inorganic S fractions, imply that deep tropical weathering led to in situ pyrite oxidation before sampling or that alteration occurred during core storage under humid conditions and sample handling. In each case, pyrite oxidation coupled with an acid attack on carbonates could promote the precipitation of Ca- and Fe-sulfate salts along fissile cleavage or voids35. The precipitation of Fe-sulfate salts implies acidic conditions (pH <7)36, under which small isotope fractionations associate with pyrite oxidation (typically <5‰)37. The extent of pyrite oxidation declines inwards from fracture surfaces, and pyrite is best preserved where encased by a silicified matrix. Our data indicate that the alteration of stratiform and matrix-supported pyrite nodules that, according to SIMS, have the lowest δ34S values led to the atypical ~9‰ δ34S difference between secondary sulfate and co-occurring sulfide (Fig. 3B). Given the prevalence of fractures adjacent to coarse pyrite forms and dissolution patterns on matrix-supported nodules and stratiform bands, the δ34SAES could be mostly derived from these pyrite, while the δ34SCRS represents an average of all pyrite present in a given sample. Such alteration can produce scattering in the LST12 and Doumé δ34S profiles but is unlikely to have overprinted the underlying stratigraphic δ34S trends. Hence, even if the Francevillian Basin is better preserved (i.e., the least metamorphosed) than most, if not all, of its Paleoproterozoic counterparts, oxidative alteration impacted the geochemistry of its strata and requires consideration. Further, given this finding, the robustness of redox-sensitive metal and trace element proxies to oxidative alteration must be evaluated similarly to assess if their signals are attributable to primary rather than secondary processes15,38,39.
Changes in pyrite morphology, texture, and chemical composition provide insight into the biogeochemical cycling of elements as sediments age. In settings rich in organic matter, framboidal and minute octahedral pyrite (type 1) with a narrow size distribution predominate in younger and more shallowly buried sediments, whereas pyrite size range and variety of textures (types 2–4) increase during burial history40,41. This coarsening trend and systematic transition from framboidal to octahedral to cubic pyrite forms primarily result from decreasing pyrite-saturated pore waters accompanying diagenesis or metamorphism42. In the LST12 and Doumé strata, pyrite nucleation began from a precursor phase that acted as a template for the later pyrite, forming successively coarser crystals or aggregates as the sediments were buried. The broad moderately to highly skewed δ34SPY distributions of the four pyrite types appear consistent with Rayleigh-type processes in increasingly isolated pore waters43.
The absolute value and range of δ34SPY data allow us to place some constraints on potential microbial fractionation and pore water geochemical evolution associated with the formation of the four pyrite types observed in our samples. We assume the starting fluids had a δ34SSO4 value of ~10‰, based on estimates for seawater sulfate from evaporites at ~2.0 Ga44. To generate the minimum δ34SPY value (-33.7 ± 6.6‰) observed in type 1 pyrite, microbial fractionation during sulfate reduction and subsequent pyrite formation (<3‰ abiotic isotope effect during pyrite precipitation)45, would have been at least ~45‰ (fractionation ε = δ34SSO4 - δ34SH2S, Eq. 1). With these constraints, we can apply the Rayleigh equation to our dataset (Fig. 6; see Materials and Methods for details). The δ34SPY distributions of all four types of pyrite textures can be reproduced by keeping the starting sulfate’s isotopic composition and net fractionation unchanged and simply varying the extent to which pore water sulfate pool was consumed. For the resulting sulfide δ34S values to exceed 10‰ (up to 53‰), a significant portion (60 – 80%) of pore water sulfate was transformed into sulfide. Considering petrographic observations, the Rayleigh-type distributions of type 1 – 4 pyrite indicate cycles of replenishment and depletion of the pore water sulfide pool after each episode of pyrite generation rather than continuous crystallization from increasingly isolated pore waters. For instance, the matrix-supported nodule’s (Fig. 5BIII) low δ34SPY (−12.6 ± 8.0‰) and octahedral habit suggest growth under high sulfide saturation consuming sulfur from a similar source as the type 2 pyrite. Conversely, the massive nodule’s (Fig. 5CIII) large size and cubic facets suggest prolonged growth at low supersaturation levels42, while the high mean δ34SPY of 20.4 ± 3.8‰ advocate for a relatively late origin from 34S-enriched fluids. Thus, we interpret the 34S-depleted type 1, 2, and 3 pyrite as having formed during intermittent yet successive mineralizing episodes until sulfur and iron sources were eventually depleted, resulting in the more 34S-enriched type 3 – 4 pyrite. Contrastingly, in the carbonate samples, nucleation of type 4 pyrite progressed gradually from less saturated solutions until at least 2/3rd of pore water sulfate was consumed. This could be due to the earlier isolation of pore waters from the overlying seawater in the carbonate beds, allowing the almost complete conversion of sulfate into sulfide while ultimately producing less pyrite than in the black shale beds. The takeaway is that the relatively high bulk δ34SCRS throughout the LST12 and Doumé cores, taken as evidence for decreasing seawater sulfate levels during the Francevillian time11, instead resulted from multiple intervals of pyrite formation under diffusion limitation that was more pervasive in the carbonate facies. No change in the abundance or isotopic composition of the parent seawater sulfate reservoir is needed to explain these data and observations.
In all lithologies, the earliest pyrite textures preserving negative δ34SPY reflect sulfide derived from organoclastic microbial sulfate reduction (MSR) that can, depending on sulfate concentrations and respiration rates, produce sulfide depleted in 34S relative to ambient sulfate by as much as ~70‰46,47. One possibility is that the smaller apparent fractionation could be a consequence of individual basin conditions (organic C, sulfate, and iron concentration, sedimentation rates, and the openness of the system) that impacted the expression of biological fractionations in the precipitating pyrite18,48. Another is that recrystallization, overgrowth, or intergrowth reduced isotopic variability by homogenizing the extreme ends of the pyrite populations δ34SPY distributions. Even so, the ~45‰ fractionation (or more) needed to produce the earliest pyrite’s δ34SPY distribution is consistent with typical microbial respiration in marine sediments41,47.
As burial progresses, the dynamics of sulfur cycling in organic-rich settings become increasingly complex, making it difficult to discern specific pyrite-generating processes. We propose that anoxic oxidation of methane coupled to sulfate reduction (AOM-SR) or (bio)degradation of organic matter, driven by increasing temperature, the reduction of Mn, Fe-oxyhydroxides or structural iron in clay minerals, induced mineralization of the paragenetically later type 2–4 pyrite49,50. Adding to early organoclastic MSR, maturing organic matter can facilitate deep subsurface methane generation and AOM-SR at temperatures <100 °C or thermochemical sulfate reduction (TSR) at >120 °C as long as soluble sulfate and organic reductants are available in the burial environment48,51,52. A ~25–65‰ kinetic isotope effect accompanies sulfate reduction during AOM-SR; the magnitude depends on ambient conditions (particularly methane concentration)53. The fractionation during TSR can range from ~25 – 40‰, although this range of fractionations is rarely preserved due to the quantitative conversion of sulfate51,54. Large fractionations only occur at the lower required temperature range for TSR, and adding to that, anhydrite dissolution often controls the rate of TSR reactions, leading to typical expressed fractionations of ~1–3‰54. Nevertheless, 34S-enriched sulfide can be generated by AOM-SR and/or TSR consuming residual pore water sulfate following organoclastic MSR, and pyrite originating from either process tends to grow coarser cubic, aggregated, or overgrowth textures41,54. Given evidence for the production of hydrocarbons8,30, differentiating and identifying specific late-diagenetic to metamorphic sulfide-generating processes in the Francevillian rocks requires careful screening of samples.
The Francevillian strata have been through the oil window and were subject to changes in stress during uplift22. Hydrofracturing related to organic matter maturation can enhance sulfate diffusivity through a shale matrix by increasing porosity or developing fractures55. For example, hydrofracturing allowed incursions of meteoric brines in the older FA and FB formations30,31,32,56 and may have generated conditions compatible with TSR33. The fissile shales and fractured carbonates with abundant pyrobitumen-filled veinlets in the studied sections suggest that these rocks underwent similar hydrofracturing. In addition to macro-scale features, on a mineral scale, the micro-scale oscillatory zonation recognized in type 2 and 3 pyrite is indicative of crystal growth under variable fluid chemistry common to hydrothermal systems57. Further, aromatic organic compounds can act as electron donors or shuttles to mediate the microbial or abiogenic reduction of high-valence metals or sulfate accompanied by the oxidation of petroleum hydrocarbons at the oil-water transition zone50. The released H2S can accumulate in pore water, back-react with organic matter, or attack Fe-bearing phases (i.e., Mn, Fe-(hydr)oxides, Fe-bearing clays, even pyrite) locally raising pyrite-saturation to form new growth layers. Since authigenic pyrite formation involves the uptake of trace elements (Mn, Co, Ni, Cu, Zn, As, Mo, and Sb) released from mineral and organic species at disparate times, characteristic trace element zonation can form as it precipitates from fluids evolving over time40. The type 2 pyrite enrichment in Cu, Co, and Ni potentially reflects their liberation from detrital components or, as recently proposed, contribution by secondary producers, i.e., methanotrophs that require Co and Ni for synthesizing enzymes58. On the other hand, As, Mo, and Mn (from degrading organic matter and reducing Mn, Fe-(hydr)oxides) were later incorporated into overgrowing type 3 pyrite (Figs. S3–6). The degradation of source kerogen is further supported by XANES spectra showing absorption peaks at 2473.7 eV and ~2481 eV, characteristic of dibenzothiophene, sulfonate, or sulfate ester that originate from oxidation of organic sulfides (Figure. S1)54. The δ34SOSC values (−2.0–30.7‰) are within <10‰ of paired δ34SCRS, as expected for equilibrium fractionations and isotope exchange with inorganic sulfide52. Together, the XANES data and the linear relationship of bulk δ34SOSC and δ34SCRS appear more consistent with kerogen maturation due to progressive burial than early diagenetic sulfurization.
Although the association between pyrobitumen and zoned pyrite does not unequivocally prove oil-mineral(-microbe) interactions, it provides a mechanism to produce secondary pools of reduced iron and sulfide favorable to the retention of pyrite in the subsurface. The cracking of early diagenetic OSCs or the introduction of external sulfate to pore waters would explain the low δ34SPY signatures of stratiform pyrite and matrix-supported nodules. The massive nodules developed distinct trace element patterns and became 34S enriched as their growth continued until the S, Fe, or other trace metals were consumed from the precipitating fluid. As such, it could be that microbial processes contributed to the formation of the earlier stratiform pyrite and matrix-supported nodules with negative δ34SPY, while the later massive nodules potentially inherited less varied and positive δ34S signatures from AOM-SR or TSR processes that eventually exhausted the residual pore water sulfate pool. Thus, the indication of hydrocarbon generation and migration preserved in the shales deposited in deeper-water settings shows how facies asserted a strong local control on sulfide production and pyrite sequestration by driving subsurface metabolic activity and/or TSR processes.
Conclusions and implications
The common understanding of C and S cycling is that the co-occurrence of low δ13Ccarb, negative δ13Corg, and positive δ34SCRS shifts in the Francevillian succession following the LJE excursion reflect complex biological and environmental feedbacks driven by high primary productivity. This process initially contributed to atmosphere-ocean oxygenation, but the accelerated flux of organic matter into the marine realm eventually depleted the oxidant pools and helped expand oceanic anoxic zones8,10,11. The observation of similar chemostratigraphic trends in other broadly correlative Paleoproterozoic sedimentary successions (e.g., the ~2.0 Ga Zaonega Formation in the Onega Basin, Karelia, Russia) has been used to argue for a global mechanism contributing to the LJE and its ultimate demise3. However, as we and others show33,54,59,60,61, using sections from petroleum-generating basins is fraught with complicating factors such as later-stage subsurface metabolic activity or TSR that can obscure primary geochemical signals and thereby lead to erroneous interpretation of chemostratigraphic trends.
Our findings also show that the C and S isotope trends observed in the Francevillian LST12 and Doumé cores reflect local sedimentary controls rather than global biogeochemical cycling processes. Instead of representing the global ocean’s isotopic composition, we interpret the facies-controlled downturns in the S-isotope trends from LST12 (FB/FC) and Doumé (FD) as the result of diagenesis progressing from different initial conditions (i.e., organic carbon loading and sediment properties controlling diffusive transport) in carbonate versus siliciclastic depositional environments. The high δ34SCRS values (>10‰) in the silicified carbonate beds can be understood as a net transition towards closed system conditions due to microbially mediated diagenetic processes. The negative shift in δ34SCRS (from ~14‰ to ~−3‰), along with coincident negative shifts in δ13Corg and low δ13Ccarb values in the studied succession representing the post-LJE interval, can be explained as due to several successive processes. Early pore water chemistry was likely dominated by MSR activity but later succeeded by AOM-SR or TSR processes driven by maturing or migrating hydrocarbon-rich fluids. Similar caution needs to be exercised when relating bulk pyrite S isotope and carbonate and organic matter C isotope records to global environmental changes, such as in the case of the Zaonega Formation3,62. Burial diagenesis and metamorphism, hydrothermal circulation, and hydrocarbon mobilization in that formation were underappreciated in previous studies and inadvertently introduced ambiguity into interpretations of these proxies59,60,61.
Our textural characterization of pyrite and sediment fabrics combined with high-resolution geochemical analysis in the Francevillian LST12 and Doumé cores, and OnZap cores of the Zaonega Formation61, demonstrates that multigenerational pyrite formation complicates relating excursions in the δ34SCRS record to fluctuations in the contemporaneous oceanic sulfate pool. In effect, the more varied the pyrite textures, the less likely bulk δ34SCRS is to reflect microbial S cycling in early pore waters. Our work also shows that early-formed pyrite originating in shallow sediments can be minor compared to subsequent generations.
To obtain reliable environmental information about depositional conditions, it is necessary to undertake and integrate traditional geologic (petrography, sedimentology) and novel geochemical approaches that yield data on different scales (i.e., wet-chemical extractions, SIMS, synchrotron X-ray, and LA-ICP-MS techniques) (Fig. 7). In doing so, the timing of mineralizing events can be inferred and thereby aid identification of geochemical proxy data that record primary depositional signals. Moreover, secondary alteration by weathering processes extending deep into the subsurface (tens of meters) in tropical regions must be carefully assessed. As we show, sulfides encased by a shale or silicified matrix can appear macroscopically to be pristine, but it is only when examined at a finer resolution do features characteristic of secondary alteration become obvious. As such, the suitability of outcrop and near-surface drill core samples for bulk-rock paleoredox investigations becomes highly suspect, as already pointed out to the geochemistry community15,63.
On the left, the coarsening trend and systematic transition from framboidal to octahedral to cubic pyrite forms are attributed to changes in pyrite-saturated pore waters accompanying burial. Continuous crystallization from increasingly isolated pore waters would lead to increasing δ34S values, while cycles of replenishment and depletion of the pore water sulfide pool after each episode of pyrite generation can result in varying δ34S of the different pyrite generations. On the right, is a best-practice protocol to follow for screening samples for paleoenvironmental investigations and interpreting bulk δ34S results.
Reliable interpretation of bulk δ34S records from pyrite requires geological context to be established via detailed field and laboratory studies focusing not only on analytical techniques but also field descriptions of textures characteristic of secondary processes (faulting, brecciation, veining/mineralization, signs of weathering, e.g., Fig. 1C). To avoid oxidative weathering alteration, samples should be primarily collected from drill cores that are screened for signs of oxidation in near-surface layers. Microscopic and spectroscopic methods can be used to study the distribution of different sulfide species and their relationships, paying attention to textures (e.g., crystal size, shape, zoning, secondary overgrowths, veins) to discriminate pyrite originating from early- and late-stage processes (e.g., Fig. 5BIV, CIV, V, DIV, V, Fig. 7, Fig. S2). After the screening of samples and defining pyrite morphologies (and those of other sulfides present), in situ investigation of δ34S (e.g., SIMS or LA-ICP-MS) of genetically distinct pyrites can reveal δ34S variability characteristic for the specific conditions under which these formed. This work will establish the context for choosing samples for wet-chemical extractions and interpreting bulk δ34S records.
Our results indicate that the most prudent interpretation of the 34S-enriched pyrite found in what is thought to be one of the best-preserved records of Paleoproterozoic Earth history, the Francevillian Basin, is that it is a product of diagenesis and late-stage processes in sulfate- and organic-rich sediments that transversed the oil window. Only the in situ δ34SPY of the earliest pyrite generation records early depositional conditions, indicating no significant changes in the MSR-associated fractionation of sulfur isotopes or isotopic composition of seawater sulfate (~10‰)44,61. While overgrowth and recrystallization may have reduced the maximum extent of biological fractionation expressed in the early diagenetic pyrite, there is no evidence to suggest that it declined to <45‰ as a response to changes in the habitat of the sulfate-reducing microbes and contraction of the seawater sulfate reservoir. This finding aligns with the interpretation of the S-isotope record from the often compared Zaonega Formation of the Onega Basin61. Both findings necessitate reevaluation of the widely used geochemical models of how Earth’s surface transitioned from an anoxic to an oxic state.
Methods
Materials and Depositional setting
Materials for this study are from the LST12 core in the Lastoursville sub-basin (drilled by Comilog-Eramet Company in 2012, 0.8089 °S, 12.7709 °E) and Doumé core (drilled in 2018 in collaboration with Tokyo Institute of Technology Earth-Life Science Institute (ELSI) and the University of Science and Technology of Masuku, Gabon, 0.8825 °S, 12.9849 °E). LST12 recovers 139 m of dolostone and black shale from the FB – FC intervals64, while the 76 m long Doumé core intersecting the FD interval is characterized by greenish to black shales and carbonates interlayered with numerous tuffs. The top 20 m in the LST12 core and top 8 m in the Doumé core show visible signs of modern weathering, e.g., the disintegration of the rocks, alteration of primary sedimentary minerals, and development of Fe-Mn oxyhydroxide coatings.
Six units I–VI are defined in core LST1212. Dolostone-dominated units I–III of LST12 represent tidal and shallow-marine deposits, while the mostly black shale units IV–VI record a transgression to depths below the storm wave base. In addition, pyrobitumen veins, fracture infills, and grains in LST12 unit III attest to hydrocarbon production and migration. LST12 units I–V that are characterized by positive carbonate carbon isotopic values have been considered as part of the LJE, and the uppermost unit VI has been interpreted as representing the decline to normal marine carbonate carbon isotopic values12.
Core Doumé is divided into three units I–III. Fine parallel-laminated, organic-rich black and calcareous shales that accumulated below the storm wave base characterize Doumé unit I. Unit II consists of alternating organic-poor grey to greenish siliceous dolomarl, shale, and tuff layers with rare sandy, cross-bedded carbonate beds. Crinkly wrinkly bed surfaces indicate microbial mat growth in these low-energy settings. The uppermost Doumé unit III is composed of parallel to wavy laminated dark grey shales interlayered with numerous tuff and occasional silt and siliceous carbonate beds, all of which show evidence of albitization. Relative to the LST12 strata, the Doumé sediments accumulated in a deeper, generally low-energy setting into which volcaniclastic and tuff beds were deposited.
Bulk chemistry and mineralogy
Core LST12 and core Doumé underwent the same sampling technique12. The mineralogical composition of 210 samples from the Doumé core was determined on unoriented powdered aliquots using a Bruker D8 Advance X-ray diffractometer using Cu Kα radiation and LynxEye position, sensitive detector in 2–70° 2 Θ range at the University of Tartu. The resulting diffractograms were interpreted and modeled using the Rietveld algorithm-based program Topaz. Relative error of quantification is better than 10% for major phases (>5 wt.%) and better than 20% for minor phases (<5 wt.%). Major and minor element compositions of 45 pulverized samples dissolved with multi-acid methods (HNO3, HClO4, HF) were determined by ICP-OES (Bureau Veritas Minerals, Canada). The average relative standard deviation was less than 5% for all elements.
Stable carbon isotope measurements
Using a subset (n = 94 for Corg, n = 52 for Ccarb) of the same sample powders used for XRD, stable C isotope ratios (δ13C) in carbonate and organic matter were measured using a Thermo Scientific Delta V Advantage and Thermo Scientific Delta V Plus continuous flow isotope ratio mass-spectrometers (IR-MS) at the University of Tartu following standard protocols. Carbonate carbon and oxygen isotopes were measured by dissolving ∼900 μg of sample in 100% phosphoric acid (H3PO4) for at least 4 h at 70 °C to produce CO2 in sealed vials. The sample vials were flushed with He to transfer the evolved CO2 to the mass spectrometer for isotope analysis. For organic carbon isotope analysis, decarbonated aliquots were obtained by treating samples with 10% (v/v) HCl for at least three rinses or until no effervescence was observed after adding the acid. The dissolution residues were rinsed with distilled water at least three times, centrifuged, and dried. Approximately 10 mg of the decarbonated samples were loaded into tin capsules for organic carbon analyses. Total organic carbon (TOC) abundance was determined from the area of the resulting CO2 peak upon combustion. All carbon isotope results are reported in standard delta notation as permil deviation from the Vienna-Pee Dee Belemnite (VPDB) standard: δ13C = (13Rsample/13RVPDB − 1) (Eq. 2). Accuracy and precision were monitored via replicate analysis of laboratory and international standards (i.e., NBS 18 δ13C = −5.01 ± 0.04‰ VPDB and IAEA-603 δ13C = 2.46 ± 0.01‰ VPDB for carbonate and IAEA-CH-3 δ13C = −24.72 ± 0.04‰ VPDB and IAEA-CH-6 δ13C = −10.45 ± 0.03‰ VPDB for organic carbon isotopes). Reproducibility was better than ± 0.2‰ for δ13Ccarb and ± 0.2‰ for δ13Corg.
Pyrite sulfur extraction
Using the chromium-reduction method65, pyrite sulfur was extracted from 33 and 29 powdered samples (~2–3 g of rock material) from the LST12 and Doumé cores, respectively. Depending on the pyrite content determined by XRD, approximately 1–0.5 g of sample powder was used in the two-step method to extract acid volatile (AVS) and chromium reducible (CRS) sulfur. In this sequential extraction method, sulfur is first liberated as H2S via hot ~25 mL 6 M HCl distillation (AVS) followed by ~25 mL of 1 M CrCl2 distillation (CRS). The samples were reacted for four hours in a specialized extraction line under an N2 atmosphere, and the resulting H2S was converted into Ag2S by bubbling into 0.1 M AgNO3. Precipitated Ag2S was rinsed three times using distilled water and then centrifuged, dried, and homogenized before analysis. The acid-insoluble residues were rinsed with distilled water at least three times or until the rinsing water ran clear and then dried and kept for subsequent organic sulfur analysis.
Extraction of sulfur bound to organic matter
Organic sulfur (OS) from acid-insoluble residues of 29 organic-rich samples (n = 14 LST12, n = 15 Doumé) was extracted by combustion with oxygen in a sealed oxygen bomb (Parr vessel). Approximately 0.2 g of sample was mixed with mineral oil and combusted beneath a water-bearing pure oxygen atmosphere in the Parr vessel. During combustion, all sulfur compounds are converted to sulfate and absorbed in a small amount of distilled water placed in the vessel, while any mineral constituents remain as ash. The combustion products were recovered with careful vessel washings, which were filtered and acidified. The filtrates were brought to a simmer, and ~1 ml of saturated BaCl2 solution was added, cooled after one hour of reaction, and left to precipitate BaSO4 over a few days. The BaSO4 precipitates were centrifuged, rinsed with distilled water at least three times, and dried for further analysis. We consider the Parr combustion extract as sulfur bound to organic matter since inorganic sulfide, elemental sulfur, and sulfate (i.e., anhydrite dissolves in heated HCl) were removed during the prior CRS extractions and, in the black shale samples, the presence of barite is unlikely.
Acid-extractable sulfate extraction
A subset of 16 samples from the LST12 and Doumé cores were subject to additional AVS distillation, intending to extract HCl dissolvable sulfate. We followed the same AVS protocol as above; however, after completion of the reaction (at least three hours), the slurries were centrifuged to separate the acid-insoluble residues from the leachate and recover the latter. The pH of the AVS leachate was then raised by slowly pipetting 6 M NaOH until periodic testing with pH test strips indicated a slightly acidic pH (6 – 7). After this step, the AVS leachate was filtered and checked for sulfate by adding ~1 ml saturated BaCl2 and, if present, recovering the BaSO4 precipitate as described above.
Sulfur isotope analysis
To determine the sulfur isotope composition of the different sulfur fractions, ~350 μg Ag2S and BaSO4 were loaded into tin capsules with excess V2O5. The 34S/32S ratio was measured on a Thermo Delta V Plus, following combustion in a Costech ECS 4010 Elemental Analyzer at Washington University in St. Louis. Sulfur isotope compositions are expressed in standard delta notation as per mil (‰) deviations from the Vienna Canyon Diablo Troilite (VCDT) standard: δ34S = (34Rsample/34RVCDT − 1) (Eq. 3). Three in-house standards cross calibrated against international standards, IAEA-S-1 (−0.3‰), IAEA-S-3 (-32.5‰), and NBS-127 (+21.1‰), were used to calibrate isotopic measurements. The measurement uncertainty was monitored throughout the analyses using check standards with well-characterized isotopic compositions: Ag2S (0.3‰ ± 0.35), BaSO4 (+14.5‰ ± 0.33), and ZnS (−5.5‰ ± 0.55). Precision was determined to be ±0.3‰ based on repeat analyses of check standards and sample replicates. Accuracy was determined to be ±0.48‰ based on the difference between measured and known values of the check standards and the long-term standard deviations of these check standards.
Secondary ion mass spectrometry
Four rock samples from shallower- to deeper-water facies were chosen from the LST12 and Doumé cores for SIMS δ34SPY measurements: LST12-79.0 (dolostone with pyrobitumen veinlets, LST12 unit III), LST12-53.8 (black shale, LST12 unit IV), LST12-31.3 (black shale with Mn-rich carbonate, LST12 unit VI) and DM-191 (black shale, Doumé unit I). The δ34SPY data reported in this study were measured using a Cameca IMS 7f-GEO SIMS instrument at Washington University in St Louis during four analytical sessions between October 2021 and July 2022, following the same procedures as in61. Scanning ion imaging mode was used to measure 34S/32S ratios of minute pyrite crystals (typically <20 μm), while spot analysis mode was used to target larger pyrite nodules. All sample mounts were coated with 60 – 70 nm of gold to prevent charging. The gold coatings were removed during pre-sputtering of the sample surface with a 3 nA Cs+ primary beam over a region that transcended the desired raster size for data acquisition (i.e., 15 × 15 μm for spot analysis and between 10 × 10 μm to 100 × 100 μm raster squares for imaging). A normal incidence electron gun was applied for charge compensation. In spot mode, a Cs+ primary beam with a 3 μm diameter and ~2.5 nA current was used for the acquisition of 32S− (0.96 sec FC1) and 34S− (4.0 sec FC2) over 12 cycles in mono-collection mode, switching between two faraday cups. For SIMS imaging, secondary ions of 32S−, 34S−, and 16O2 were recorded using an electron multiplier (EM) detector and employing a 1 μm and 7–18.5 pA Cs+ primary beam for 60 – 450 cycles of acquisition. The spectrometer entrance slit was adjusted so that each session’s maximum instantaneous count rate was constrained to ~250,000 cps.
The SIMS images were processed with CAMECA software, and the raw isotope ratios for pyrite crystals were calculated across a region of interest (ROI) by integrating the 34S/32S ion count ratio over all cycles of data acquisition. To target pristine pyrite grains without signs of oxidation, the ROI’s (~0.5 – 1500 μm2 sizes) boundaries were defined by a threshold in the 32S− signal, avoiding regions with 16O2, and comparisons with SEM BSE images of the corresponding pyrite crystals. The raw isotope ratios were then extracted to Microsoft Excel, where additional corrections, including dead-time and QSA, were applied to the data66. The isotope ratios from unknown pyrite were normalized to the embedded in-house pyrite standard (calibrated against Balmat pyrite)67. Measurements of the unknown pyrite were bracketed between analyses of the in-house standard during the same analytical session. The standard error (1σ) for spot analysis averages 0.4‰ while, depending on the ROI’s size, the relative standard error (RSE) for ion imaging varies between 0.1 – 9.5‰ (average 1.6‰).
µXRF and XANES
A combination of µ-X-ray Fluorescence (µ-XRF) imaging and X-ray Absorption Near Edge Structure (XANES) spectroscopy was used to generate chemical speciation maps of sulfur at the GeoSoilEnviroCARS microprobe beamline 13-ID-E at the Advanced Photon Source (APS), Argonne National Laboratory, and beamline 14-3 at the Stanford Synchrotron Radiation Lightsource (SSRL), Stanford Linear Accelerator Center. Detailed descriptions of each beamline and measurement approach are found in68. At the APS, an anhydrite standard provides the white line for sulfate (2481.5 eV), and the µ-XRF maps were collected at the sulfide and sulfate excitation energies at 2472 and 2481.5 eV, respectively. At SSRL, a sodium thiosulfate powder calibrates the S K-edge to 2472.02 eV. To detect differences in organic and inorganic species, maps were collected at 2471.8, 2473.7, 2477.8, 2480.7, and 2482.4 eV that correspond to the sulfide, thiol, sulfoxide, sulfonate, and sulfate excitation energies, respectively.
The μ-XRF maps were used to determine the locations for S K-edge XANES analysis and verify speciation. The XANES spectra from both the APS and SSRL were processed using the Athena software69. All spectra were normalized using a background subtraction of the linearized pre- and post-edge region (2465 and 2510 eV, respectively). As noted in previous studies68, comparing the standards XANES spectra at each beamline, the SSRL data are systematically shifted to higher energies (by 0.5 eV) than the APS data due to calibration differences. To correct the energy differences and align the APS and SSRL energy scales, pyrite in all sample sets was calibrated to 2471.8 eV as measured during the analytical session in July 2021 at SSRL (APS data were shifted by +0.5 eV).
Identifying S species from XANES spectra
Measured XANES spectra of natural samples commonly represent mixtures of S species. The number of component species for each sample was determined by using principal component analysis (PCA) and quantified by performing a linear combination fit (LCF) with spectra of reference compounds from in-house results: pyrite, anhydrite, and barite standards, and pyrobitumen veins were used as endmembers. In addition, sphalerite, pyrrhotite, gypsum, Na- and Fe-sulfate, and various OSCs (disulfide, methionine, dibenzothiophene, sulfoxide, sulfone, sulfonate, sulfate ester) from SSRL and APS internal databases, and the European Synchrotron Radiation Facility (ESRF) database (https://www.esrf.fr/home/UsersAndScience/Experiments/XNP/ID21/php.html) were used in the LCF. However, deconvolving S species with LCF method is inaccurate for spectra with lower signal-to-noise ratios or at overlapping energy ranges.
The S XANES spectra from our 8 samples are generally characterized by several distinct signals at the energy regions representing inorganic metal sulfides (~2469–2473.4 eV), aromatic sulfur (2473.7 eV), oxidized organic sulfur (2480.8–2482.4 eV), and inorganic sulfate (2482.1–2482.4 eV). Pyrite was the dominant inorganic sulfide (2471.8 eV) in all samples, while Fe-monosulfide (at 2469.7 eV) and Zn-sulfide (at 2473.4 eV) were present in minor amounts (Fig. S1). Spectra with high signal-to-noise ratios allowed identification of various sulfate minerals (Ba-, Fe-, and Ca-sulfate); however, the lower energy shoulder of the primary sulfate peak or lower signal-to-noise ratios, complicated assigning the prominent features at ~2482 eV to a specific inorganic sulfate mineral confidently. Thus, we consider the energy region 2482.1–2482.4 eV characteristic of inorganic sulfate.
The additional XANES spectroscopy features between the inorganic sulfide and sulfate endmembers best resemble reduced, aromatic, and oxygenated S species, such as organic monosulfide, aromatic dibenzothiophene, sulfone, sulfonate or sulfate ester. The prominent feature at 2473.7 eV from pyrobitumen is similar to dibenzothiophene70 and makes up the largest portion of OSC’s in our samples. Aromatic hydrocarbons, including dibenzothiophenes, have previously been detected from the FB black shales near Oklo and are considered indicative of an upper oil window thermal maturity56. The most likely candidates for displaying a primary feature at 2473.4 eV are Zn-sulfide, disulfide, or organic monosulfide. The presence of Zn-sulfide was confirmed by other geochemical methods (SEM-EDS, LA-ICP-MS); thus, the primary feature at 2473.4 eV is assigned to Zn-sulfide. Given the close energies between sulfonate, sulfate esters, and inorganic sulfate, it can be difficult to distinguish the detection of a sulfate ester versus inorganic sulfate with a sulfonate shoulder. Thus, instead of a specific compound, we interpret the lower energy shoulder of the primary sulfate peak as belonging to an oxidized OSC.
Laser ablation inductively coupled mass spectrometry
To determine the cause of the chemical zonation in the SEM backscatter intensity images, in situ element mapping of three polished samples LST12-31.3, LST12-53.8, and LST12-61.18 (black shale, LST12 unit IV) was conducted by using laser ablation inductively coupled mass spectrometry (LA-ICP-MS) at the University of Tartu. Trace element maps were generated for the zoned type 2 pyrite in stratiform bands (LST12-53.8, LST12-61.18) and type 3 nodule pyrite (LST12-31.3, LST12-53.8). Trace element measurements were performed using a Cetac LSX-213 G2+ laser system with a HelEx II fast-washout two-volume large-format cell using 800 ml/min helium as carrier gas. The composition of the ablated material was analyzed using an Agilent 8800 quadrupole ICP-MS in single quad mode. To produce the maps, sets of parallel laser lines across areas containing zoned pyrite were scanned with a 10 μm beam size, line spacing of 10 μm, 2 μm/s scan speed at 10 Hz, and fluence of 3 J/cm2. For samples LST12-53.8 and LST12-61.18, the following masses were measured with corresponding dwell times 34S, 57Fe, 197Au–5 ms, 55Mn, 66Zn, 208Pb–10 ms, and 59Co, 60Ni, 63Cu, 75As, 95Mo, 121Sb–19 ms. For sample LST12-31.3 the masses and dwell times were 13C, 201Hg–10 ms, 34S–10.8 ms, and 47Ti, 57Fe, 59Co, 60Ni, 63Cu, 66Zn, 75As, 95Mo, 121Sb–14 ms. At the beginning and end of each mapped area, USGS GSD-1 (30 μm spot, 5 μm/s) and MASS-1 (20 μm spot, 30 μm/s) reference materials were ablated by adjusting spot size and scan speed to match signal intensities with samples, to correct for instrumental drift, and quantify element concentrations. Elemental concentrations were calculated using Iolite 4 software package and Trace Elements DRS71 from raw spectrometry data. Element maps were compiled and processed by setting 34S as the internal standard element, assuming a stoichiometric pyrite S concentration of 53.45%, and using MASS-1 as the primary reference material. Resultant element concentration maps are shown using a linear color scale.
The TE maps from LST12-53.8 (Fig. S3, S4) and LST12-61.18 (Fig. S5) reveal distinct distribution patterns for Mn, Co, Ni, Cu, Zn, As, Mo, and Sb across stratiform pyrite and massive pyrite nodules. The individual type 2 pyrite (octahedral crystals) and its enclosed remnants in both the cemented stratiform bands (Fig. S3) or nodules (developing cubic facets) exhibit oscillatory zonation of Mn, Co, Ni, Cu, As, Mo, and Sb from the core to the rim. Specifically, Cu is concentrated in the cores of type 2 pyrite, while Co, Ni, and Sb abundances are high both in the cores of the crystals and edges of the stratiform bands and nodules. Notably, there is a ten-fold increase in As concentrations accompanied by enrichments of Mn and Mo in the overgrowing type 3 pyrite forming cements and nodules. The irregular distribution of Zn suggests that it mainly occurs as a distinct phase. The trace element distribution patterns from sample LST12-31.3 (Fig. 5, Fig. S6) matrix-dominated pyrite nodule are not as defined; however, as in the other samples, Cu is concentrated in the finer pyrite crystals, Co, Ni, and As mainly occur towards the edges of pyrite masses, while Mo is concentrated in the coarser crystals and masses.
Model setup and parameters
In the studied samples, the type 1 pyrite carries low δ34SPY values (lowest −33‰), characteristic of MSR in shallow sediments, and all four pyrite type’s broad moderate to high right-skewed δ34SPY distributions indicate Rayleigh-type processes in increasingly isolated pore waters43. In the context of our samples, Rayleigh behavior of the S isotopes does not necessarily describe a closed system. On the contrary, extensive diffusion exchange with the overlying water column is required to sustain high sulfide saturation for octahedral crystal and abundant pyrite growth (up to 14 wt.%) in the organic-rich sediments. Nevertheless, applying the Rayleigh equation to our data set allows estimating a closed system endmember as follows:
where δ34S0 is the starting and δ34Sr remaining sulfate’s value, αnet is the net fractionation factor, and ƒ is the fraction of sulfate remaining in the sulfate reservoir after a portion of it has been reduced by MSR and buried as reduced sulfur. The δ34SH2S of instantaneous sulfide can be calculated according to the following:
The relationship between αnet and ε (isotope fractionation between two reservoirs in per mil) can be expressed as follows:
For the unknown parameters, we assume δ34S0 of 10‰ for seawater sulfate at ~2.0 Ga44. The net fractionation was set at 45‰ by subtracting from the δ34S0 the lowest measured -33.7 ± 6.6‰ δ34SPY from the type 1 pyrite. The maximum extent of biological fractionation is unlikely to be expressed in our samples. Even the early diagenetic type 1 pyrite formed under some diffusion limitation as it nucleated on a precursor phase. In addition, recrystallization and intergrowth likely erased the extreme ends of the original δ34S range of the pyrite textures. Thus, it is entirely possible that αnet was greater, but for types 1–3 pyrite, fractionations above 40‰ and for type 4 above 30‰ are required to reproduce the measured δ34SPY ranges (Supplementary Data 6). In the context of our samples, an igneous ~0‰ sulfur source72 is unlikely, and the source sulfate for TSR from hydrothermal fluids or evaporite minerals would likely have a seawater-like isotopic composition.
Data availability
The authors declare that data supporting the findings of this study are available within the paper, its supplementary information files, and the Figshare repository. https://doi.org/10.6084/m9.figshare.24785643.v1.
References
Canfield, D. E. The evolution of the Earth surface sulfur reservoir. Am. J. Sci. 304, 839–861 (2004).
Johnston, D. T. Multiple sulfur isotopes and the evolution of Earth’s surface sulfur cycle. Earth-Sci. Rev. 106, 161–183 (2011).
Kump, L. R. et al. Isotopic evidence for massive oxidation of organic matter following the great oxidation event. Science 334, 1694–1696 (2011).
Holland, H. D. The oxygenation of the atmosphere and oceans. Philos. T R. Soc. B 361, 903–915 (2006).
Hodgskiss, M. S. W. & Sperling, E. A. A prolonged, two-step oxygenation of Earth’s early atmosphere: Support from confidence intervals. Geology 50, 158–162 (2022).
Gauthier-Lafaye, F. & Weber, F. Natural nuclear fission reactors: Time constraints for occurrence, and their relation to uranium and manganese deposits and to the evolution of the atmosphere. Precambrian Res. 120, 81–100 (2003).
Canfield, D. E. et al. Oxygen dynamics in the aftermath of the Great Oxidation of Earth’s atmosphere. Proc. Natl Acad. Sci. 110, 16736–16741 (2013).
Ossa Ossa, F. et al. Limited expression of the Paleoproterozoic Oklo natural nuclear reactor phenomenon in the aftermath of a widespread deoxygenation event ~2.11–2.06 billion years ago. Chem. Geol. 578, 120315 (2021).
Bonhomme, M. G., Gauthier-Lafaye, F. & Weber, F. An example of lower proterozoic sediments: The Francevillian in Gabon. Precambrian Res. 18, 87–102 (1982).
Bekker, A. & Holland, H. D. Oxygen overshoot and recovery during the early Paleoproterozoic. Earth Planet. Sci. Lett. 317–318, 295–304 (2012).
Ossa Ossa, F. et al. Two-step deoxygenation at the end of the Paleoproterozoic Lomagundi Event. Earth Planet. Sci. Lett. 486, 70–83 (2018).
Mayika, K. B. et al. The Paleoproterozoic Francevillian succession of Gabon and the Lomagundi-Jatuli event. Geology 48, 1099–1104 (2020).
Prave, A. R. et al. The grandest of them all: the Lomagundi–Jatuli Event and Earth’s oxygenation. J. Geol. Soc. 179, jgs2021–jgs2036 https://doi.org/10.1144/jgs2021-036 (2022).
Hodgskiss, M. S. W., Crockford, P. W. & Turchyn, A. V. Deconstructing the Lomagundi-Jatuli Carbon Isotope Excursion. Annu. Rev. Earth Planet. Sci. 51, 301–330 (2023).
Ahm, A.-S. C., Bjerrum, C. J. & Hammarlund, E. U. Disentangling the record of diagenesis, local redox conditions, and global seawater chemistry during the latest Ordovician glaciation. Earth Planet. Sci. Lett. 459, 145–156 (2017).
Jones, D. S. et al. Sea level, carbonate mineralogy, and early diagenesis controlled δ13C records in Upper Ordovician carbonates. Geology 48, 194–199 (2020).
Liu, J. et al. Early diagenesis of iron and sulfur in Bornholm Basin sediments: The role of near-surface pyrite formation. Geochim. et Cosmochim. Acta 284, 43–60 (2020).
Pasquier, V., Bryant, R. N., Fike, D. A. & Halevy, I. Strong local, not global, controls on marine pyrite sulfur isotopes. Sci. Adv. 7, eabb7403 (2021).
Weber, F. Une série précambrienne du Gabon: le Francevillien. Sédimentologie, géochimie, relations avec les gites minéraux associés. Mémoires du Service de la Cart Géologique d'Alsace et de Lorraine. 28, 328 p (1968).
Thiéblemont, D. et al. Notice Explicative de La Carte Géologique de La République Du Gabon à 1/200 000, Feuille Fougamou. Editions DGMG – Ministères Des Mines, Du Pétrole, Des Hydrocarbures. Libreville 63 p (2009).
Gauthier-Lafaye, F. Les Gisements d’uranium Du Gabon et Les Réacteurs d’Oklo: Modèle Métallogénique de Gîtes a Fortes Teneurs Du Protérozoïque Inférieur. (Université Louis Pasteur, Bibliothèque de l’Institut de Géologie, Strasbourg: 1986).
Weber, F., Gauthier-Lafaye, F., Whitechurch, H., Ulrich, M. & El Albani, A. The 2-Ga Eburnean Orogeny in Gabon and the opening of the Francevillian intracratonic basins: A review. Comptes Rendus Geosci. 348, 572–586 (2016).
Sawaki, Y. et al. Chronological constraints on the Paleoproterozoic Francevillian Group in Gabon. Geosci. Front. 8, 397–407 (2017).
Horie, K., Hidaka, H. & Gauthier-Lafaye, F. Goldschmidt Conference Abstracts 2005. Geochim. et Cosmochim. Acta 69, 4–50 (2005).
Liu, W. & Zhang, X. Experimental study of microbial pyrite oxidation: a suggestion for geologically useful biosignatures. Geomicrobiol. J. 32, 466–471 (2015).
Igathinathane, C., Pordesimo, L. O., Columbus, E. P., Batchelor, W. D. & Methuku, S. R. Shape identification and particles size distribution from basic shape parameters using ImageJ. Comput. Electron. Agric. 63, 168–182 (2008).
Ossa Ossa, F. et al. Moderate levels of oxygenation during the late stage of Earth’s Great Oxidation Event. Earth Planet. Sci. Lett. 594, 117716 (2022).
El Albani, A. et al. The 2.1 Ga old Francevillian biota: biogenicity, taphonomy and biodiversity. PLoS ONE 9, e99438 (2014).
El Albani, A. et al. Large colonial organisms with coordinated growth in oxygenated environments 2.1 Gyr ago. Nature 466, 100–104 (2010).
Gauthier-Lafaye, F. & Weber, F. The Francevillian (Lower Proterozoic) uranium ore deposits of Gabon. Econ. Geol. 84, 2267–2285 (1989).
Mathieu, R., Cuney, M. & Cathelineau, M. Geochemistry of palaeofluids circulation in the Franceville basin and around Oklo natural nuclear reaction zones (Gabon). J. Geochem. Explor. 69–70, 245–249 (2000).
Mathieu, R., Zetterström, L., Cuney, M., Gauthier-Lafaye, F. & Hidaka, H. Alteration of monazite and zircon and lead migration as geochemical tracers of fluid paleocirculations around the Oklo–Okélobondo and Bangombé natural nuclear reaction zones (Franceville basin, Gabon). Chem. Geol. 171, 147–171 (2001).
Lecomte, A. et al. Uranium deposits of Franceville basin (Gabon): Role of organic matter and oil cracking on uranium mineralization. Ore Geol. Rev. 123, 103579 (2020).
Braun, J.-J. et al. Present weathering rates in a humid tropical watershed: Nsimi, South Cameroon. Geochim. et Cosmochim. Acta 69, 357–387 (2005).
Gu, X., Heaney, P. J., Reis, F. D. A. A. & Brantley, S. L. Deep abiotic weathering of pyrite. Science 370, eabb8092 (2020).
Todd, E. C., Sherman, D. M. & Purton, J. A. Surface oxidation of pyrite under ambient atmospheric and aqueous (pH = 2 to 10) conditions: electronic structure and mineralogy from X-ray absorption spectroscopy. Geochim. et Cosmochim. Acta 67, 881–893 (2003).
Balci, N., Shanks, W. C., Mayer, B. & Mandernack, K. W. Oxygen and sulfur isotope systematics of sulfate produced by bacterial and abiotic oxidation of pyrite. Geochim. et Cosmochim. Acta 71, 3796–3811 (2007).
Thomson, J. et al. Redistribution and geochemical behaviour of redox-sensitive elements around S1, the most recent eastern Mediterranean sapropel. Geochim. et Cosmochim. Acta 59, 3487–3501 (1995).
Chen, C. et al. New evidence for compaction-driven vertical fluid migration into the Upper Ordovician (Hirnantian) Guanyinqiao bed of south China. Palaeogeogr., Palaeoclimatol., Palaeoecol. 550, 109746 (2020).
Large, R. R., Maslennikov, V. V., Robert, F., Danyushevsky, L. V. & Chang, Z. Multistage sedimentary and metamorphic origin of pyrite and gold in the Giant Sukhoi Log deposit, Lena Gold Province, Russia. Economic Geol. 102, 1233–1267 (2007).
Liu, J. et al. Multiple sulphur isotopes discriminate organoclastic and methane-based sulfate reduction by sub-seafloor pyrite formation. Geochim. et Cosmochim. Acta S0016703721005688 (2021) https://doi.org/10.1016/j.gca.2021.09.026.
Wang, Q. & Morse, J. W. Pyrite formation under conditions approximating those in anoxic sediments I. Pathway and morphology. Mar. Chem. 52, 99–121 (1996).
Ohmoto, H., Kakegawa, T. & Lowe, D. R. 3.4-billion-year-old biogenic pyrites from Barberton, South Africa: Sulfur isotope evidence. Science 262, 555–557 (1993).
Blättler, C. L. et al. Two-billion-year-old evaporites capture Earth’s great oxidation. Science eaar2687 https://doi.org/10.1126/science.aar2687 (2018).
Böttcher, M. E., Smock, A. M. & Cypionka, H. Sulfur isotope fractionation during experimental precipitation of iron(II) and manganese(II) sulfide at room temperature. Chem. Geol. 146, 127–134 (1998).
Sim, M. S., Bosak, T. & Ono, S. Large sulfur isotope fractionation does not require disproportionation. Science 333, 74–77 (2011).
Wing, B. A. & Halevy, I. Intracellular metabolite levels shape sulfur isotope fractionation during microbial sulfate respiration. Proc. Natl Acad. Sci. 111, 18116–18125 (2014).
Jørgensen, B. B., Bottcher, M. E., Luschen, H., Neretin, L. N. & Volkov, I. I. Anaerobic methane oxidation and a deep H2S sink generate isotopically heavy sulfides in Black Sea sediments. Geochim. et Cosmochim. Acta 68, 2095–2118 (2004).
Kelemen, S. R., Sansone, M., Walters, C. C., Kwiatek, P. J. & Bolin, T. Thermal transformations of organic and inorganic sulfur in Type II kerogen quantified by S-XANES. Geochim. et Cosmochim. Acta 83, 61–78 (2012).
Hu, W.-X. et al. Thermochemical oxidation of methane induced by high-valence metal oxides in a sedimentary basin. Nat. Commun. 9, 5131 (2018).
Kiyosu, Y. Chemical reduction and sulfur-isotope effects of sulfate by organic matter under hydrothermal conditions. Chem. Geol. 30, 47–56 (1980).
Amrani, A. Organosulfur compounds: molecular and isotopic evolution from biota to oil and gas. Annu Rev. Earth Pl Sc. 42, 733–768 (2014).
Deusner, C. et al. Sulfur and oxygen isotope fractionation during sulfate reduction coupled to anaerobic oxidation of methane is dependent on methane concentration. Earth Planet. Sci. Lett. 399, 61–73 (2014).
Cai, C., Li, H., Li, K. & Wang, D. Thermochemical sulfate reduction in sedimentary basins and beyond: A review. Chem. Geol. 607, 121018 (2022).
Teixeira, M. G. et al. Microfracturing during primary migration in shales. Tectonophysics 694, 268–279 (2017).
Dutkiewicz, A., George, S. C., Mossman, D. J., Ridley, J. & Volk, H. Oil and its biomarkers associated with the Palaeoproterozoic Oklo natural fission reactors, Gabon. Chem. Geol. 244, 130–154 (2007).
Wu, Y.-F. et al. Gold, arsenic, and copper zoning in pyrite: A record of fluid chemistry and growth kinetics. Geology 47, 641–644 (2019).
Chen, C. et al. Sulfate-driven anaerobic oxidation of methane inferred from trace-element chemistry and nickel isotopes of pyrite. Geochim. et Cosmochim. Acta 349, 81–95 (2023).
Črne, A. E. et al. Petrography and geochemistry of carbonate rocks of the Paleoproterozoic Zaonega Formation, Russia: Documentation of C-13-depleted non-primary calcite. Precambrian Res. 240, 79–93 (2014).
Kreitsmann, T. et al. Hydrothermal dedolomitisation of carbonate rocks of the Paleoproterozoic Zaonega Formation, NW Russia — Implications for the preservation of primary C isotope signals. Chem. Geol. 512, 43–57 (2019).
Paiste, K., Fike, D. A., Kirsimäe, K., Jones, C. & Lepland, A. Testing the global significance of the sulfur isotope record of the ca. 2.0 Ga Zaonega Formation: A micro-scale S isotope investigation. Geochim. et Cosmochim. Acta 331, 86–104 (2022).
Scott, C. et al. Pyrite multiple-sulfur isotope evidence for rapid expansion and contraction of the early Paleoproterozoic seawater sulfate reservoir. Earth Planet Sc. Lett. 389, 95–104 (2014).
Planavsky, N. J., Robbins, L. J., Kamber, B. S. & Schoenberg, R. Weathering, alteration and reconstructing Earth’s oxygenation. Interface Focus 10, 20190140 (2020).
Préat, A., Weber, F. & Comment on Ossa Ossa. et al. (2018) paper published in EPSL. Earth and Planetary. Sci. Lett. 511, 256–258 (2019).
Canfield, D. E., Raiswell, R., Westrich, J. T., Reaves, C. M. & Berner, R. A. The use of chromium reduction in the analysis of reduced inorganic sulfur in sediments and shales. Chem. Geol. 54, 149–155 (1986).
Jones, C., Fike, D. A. & Meyer, K. M. Secondary Ion Mass Spectrometry methodology for isotopic ratio measurement of micro-grains in thin sections: true grain size estimation and deconvolution of inter-grain size gradients and intra-grain radial gradients. Geostand. Geoanal Res. 43, 61–76 (2019).
Crowe, D. E. & Vaughan, R. G. Characterization and use of isotopically homogeneous standards for in situ laser microprobe analysis of 34 S/ 32 S ratios. Am. Min. 81, 187–193 (1996).
Richardson, J. A. et al. The source of sulfate in brachiopod calcite: Insights from μ-XRF imaging and XANES spectroscopy. Chem. Geol. 529, 119328 (2019).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Raven, M. R. et al. Organic matter sulfurization and organic carbon burial in the Mesoproterozoic. Geochim. et Cosmochim. Acta 347, 102–115 (2023).
Paton, C., Hellstrom, J., Paul, B., Woodhead, J. & Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. Spectrom. 26, 2508 (2011).
Ono, S., Shanks, W. C., Rouxel, O. J. & Rumble, D. S-33 constraints on the seawater sulfate contribution in modern seafloor hydrothermal vent sulfides. Geochim. et Cosmochim. Acta 71, 1170–1182 (2007).
Acknowledgements
We gratefully acknowledge J. L. Houghton for assistance with laboratory and SIMS procedures at Washington University in St Louis. We thank M. Newille for his guidance and helpful advice at the Advanced Photon Source (APS). M. R. Raven is thanked for sharing XANES spectra from organic sulfur standards, J. G. Catalano for useful advice regarding LCF of XANES, and P. Paiste for assistance with LA-ICP-MS measurements. We appreciate the constructive commentary provided by three anonymous reviewers.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 894831. D.A.F. received funding from Washington University’s McDonnell Center for the Space Sciences. Y.S. and T.S. were supported by JSPS KAKENHI Grant Numbers 17H04858 and 19K14828, respectively. K.K. received funding from the Estonian Science Agency project PRG2123. The analyses at the University of Tartu, Estonia, were supported by the Estonian Centre of Analytical Chemistry. Portions of this work were performed at GeoSoilEnviroCARS (The University of Chicago, Sector 13), Advanced Photon Source (APS), and Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation – Earth Sciences (EAR – 1634415). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P30GM133894). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Author information
Authors and Affiliations
Contributions
K.P., K.B.M., K.K., J.A.R., R.S.W., C.J., and S.A.W. carried out the chemical analysis and data quality control. K.P., K.B.M., K.K., D.A.F., A.L., M.M., T.S., Y.U., and Y.S. contributed to the interpretations. K.P., D.A.F., K.K., A.L., and A.R.P. conceived the work and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Jiasheng Wang, Benjamin Johnson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Carolina Ortiz Guerrero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paiste, K., Fike, D.A., Mayika, K.B. et al. Sulfur isotopes from the Paleoproterozoic Francevillian Basin record multigenerational pyrite formation, not depositional conditions. Commun Earth Environ 5, 328 (2024). https://doi.org/10.1038/s43247-024-01498-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-024-01498-1
- Springer Nature Limited