Abstract
The pupillary light reflex (PLR) adapts the amount of light reaching the retina, protecting it and improving image formation. Two PLR mechanisms have been described in vertebrates. First, the pretectum receives retinal inputs and projects to the Edinger-Westphal nucleus (EWN), which targets the ciliary ganglion through the oculomotor nerve (nIII). Postganglionic fibers enter the eye-globe, traveling to the iris sphincter muscle. Additionally, some vertebrates exhibit an iris-intrinsic PLR mechanism mediated by sphincter muscle cells that express melanopsin inducing muscle contraction. Given the high degree of conservation of the lamprey visual system, we investigated the mechanisms underlying the PLR to shed light onto their evolutionary origins. Recently, a PLR mediated by melanopsin was demonstrated in lampreys, suggested to be brain mediated. Remarkably, we found that PLR is instead mediated by direct retino-iridal cholinergic projections. This retina-mediated PLR acts synergistically with an iris-intrinsic mechanism that, as in other vertebrates, is mediated by melanopsin and has contribution of gap junctions between muscle fibers. In contrast, we show that lampreys lack the brain-mediated PLR. Our results suggest that two eye-intrinsic PLR mechanisms were present in early vertebrate evolution, whereas the brain-mediated PLR has a more recent origin.
Similar content being viewed by others
Introduction
Pupil contraction through the pupillary light reflex (PLR) reduces the amount of light that reaches the retina, protecting it and maximizing image formation efficiency1,2. In vertebrates, two different PLR mechanisms have been described. In the first one, the retina sends light information through the optic nerve (nII) to the pretectum, a brain area that, in turn, projects to the Edinger-Westphal nucleus (EWN). Then, the EWN sends signals to the ciliary ganglion through the oculomotor nerve (nIII), and efferent fibers from the ciliary ganglion enter the ocular globe, traveling to the iris sphincter muscle and releasing acetylcholine (ACh)1. Additionally, fish, amphibians, birds, and nocturnal/crepuscular mammals exhibit a PLR mechanism that is intrinsic to the iris, mediated by sphincter muscle cells that express melanopsin and can thus act as photoreceptors and evoke muscle contracion3,4,5,6. These melanopsin-expressing muscle fibers are in turn connected to adjacent muscle fibers via gap-junctions, allowing the spread of the excitability changes evoked by light5. In mice, it has been shown that blockade of cholinergic transmission results in a significant decrease of the eye-intrinsic PLR (iPLR), and that damage to the ciliary marginal zone also has an impact in mice iPLR7, and these authors also suggest the possibility of direct communication between intrinsically photosensitive retinal ganglion cells (ipRGCs) in the retina and post-synaptic sites in the iris. However, this mechanism is controversial, and the precise pathway has not been determined yet5. Moreover, the evolutionary origin of the PLR mechanisms is also unknown. Recently, it has been demonstrated that lampreys, belonging to the oldest group of extant vertebrates, also exhibit PLR that is mediated by melanopsin8. Preliminary experiments suggested that the lamprey PLR is brain-mediated since it could not be evoked in isolated eyes8. Lampreys have a well-developed visual system with image-forming camera eyes, and an organization of extraocular muscles remarkably similar to that of other vertebrates. Moreover, the main visual centers in the brain found in other vertebrates are also present in lampreys, including the pretectum, the optic tectum, and a primordial visual cortex9,10,11,12,13,14. Therefore, we investigated the mechanisms underlying the PLR to shed light on their origin and evolution in vertebrates. Remarkably, we found that the PLR is mediated by direct cholinergic projections from the retina to the iris. Additionally, a second mechanism, intrinsic to the iris, also contributes synergistically to the PLR. This iris-intrinsic mechanism is mediated by melanopsin, and gap junctions contribute to the spread of the excitability, therefore being like the mechanism reported in other vertebrates5. However, we here show that the brain-mediated PLR is not present in lampreys. Our results therefore suggest that two PLR mechanisms intrinsic to the eye were present at the dawn of vertebrate evolution and were conserved in some vertebrate groups, whereas the brain-mediated PLR is evolutionarily more recent.
Results
Characterization of the lamprey iris sphincter muscle
The presence of a PLR in lampreys8,15 implied that intraocular muscles would also exist, but their presence had only been suggested in the species Mordacia mordax16. Sagittal sections of the eye showed that the iris sphincter muscle is formed by a thin layer of 2–3 fusiform cells adjacent to the external side of the pigmented layer of the iris (Fig. 1a–f) in the two species analyzed, Petromyzon marinus and Lampetra fluviatilis. In Fig. 1c, a photomicrograph showing the iris structure of a representative Petromyzon marinus specimen can be observed. Two epithelial layers form the posterior part of the iris, one of them pigmented. Next, a layer of connective tissue that also has pigmentation, and then the layer of muscle. The muscle nature of this layer was confirmed by Gömöri Trichrome staining, which is specific to differentiate muscle (Fig. 1d). The anterior layer external to the muscle is the stroma17,18,19; Fig. 1c–f). The muscle layer in the iris starts near the pupil and extends to the iris root near the retina (see arrows in Fig. 1e, f). Transmission electron microscopy confirmed that numerous myofilament fiber bundles are present in these cells20 (Fig. 1g, h), further confirming that they are muscle fibers. In Fig. 1g, longitudinal fibers can be seen, whereas in Fig. 1h both longitudinal and transversally cut fibers can be seen at higher magnification.
a Hematoxilin-eosin stained sagittal section of the eye, showing the overall anatomy and location of the iris sphincter muscle. b Detail of the photomicrograph shown in a, indicating the location of the sphincter muscle fibers in the iris (red arrowheads). c Toluidin blue stained sagittal section of the eye showing that the iris sphincter muscle is formed by a thin layer of fibers close to the pigmented layer of the iris. d Same structures are shown using Gömöry’s trichrome staining. The iris muscle (IM) is formed by a thin layer of fibers whose nuclei can be seen in a darker color (green arrowhead). Note that the connective tissue layer (CTL) is broken due to sectioning. e Toluidin blue stained iris showing the muscle (red arrowheads) limit in the iris near the retina. f Toluidin blue stained iris showing the muscle (red arrowheads) limit near the pupil margin. g Transmission electron microscopy image showing the presence of abundant bundles of myosin fibers (red arrowheads). h Detail of longitudinal (red arrowhead) and transverse (green arrowhead) myofilaments in an iris muscle cell using transmission electron microscopy. All images belong to P. marinus specimens, except the Gömöry’s trichrome staining in d, performed on L. fluviatilis. Scale bars = 250 µm in a; 50 µm in b; 100 µm in c, d and f; 200 µm in e; 0.2 µm in g and 100 nm in h. PEL pigmented epithelial layer, EL epithelial layer, M mitochondrion, N nucleus.
Lampreys lack a brain-mediated PLR
Lampreys have a highly conserved visual system9,10,11,12,13,14, and it has been suggested that their PLR is mediated by the brain8. Thus, we first investigated whether the pretectum-Edinger-Westphal nucleus pathway is also present in lampreys. We used an isolated eye/brain preparation to monitor iris contraction while manipulating/stimulating brain regions of interest9,12,21. In this preparation, reliable pupil contraction (tracked with DeepLabCut22) was evoked by presenting light with an LED to one eye (Fig. 2a). The PLR was analyzed in P. marinus, and L. fluviatilis (Fig. 2b–f; Supplementary Movie 1) and contraction rates were like those previously reported in intact animals8. Pupil contraction was somewhat smaller in L. fluviatilis (Fig. 2e, f), but since no obvious differences were observed between the two species, the mechanisms described apply to both.
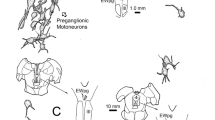
a Experimental preparation used to investigate the role of the brain in the PLR. b Normalized pupil area in response to light stimulation in the intact preparation (black line), after sectioning both oculomotor nerves (nIII, yellow), and after brain removal (red), in a representative Petromyzon marinus specimen (paired t-test p = 1). c Normalized averaged data (mean ± SD) for pupil area in response to light stimulation in the intact preparation (black line), after bilateral nIII sectioning (yellow), and after brain removal (red) in Petromyzon marinus (N = 4, paired t-test p = 1). d Frames showing pupil size before (top) and after (bottom) light stimulation, in a representative animal before (left) and after brain removal (right). e Normalized pupil area in response to light stimulation in the intact preparation (black line), and after brain removal (red), in a representative Lampetra fluviatilis specimen (paired t-test p = 0.734). f Normalized averaged data (mean ± SD) for pupil area in response to light stimulation in the intact preparation (black line), and after brain removal (red) in Lampetra fluviatilis (N = 3; paired t-test p = 1). g Representative frames showing no changes in pupil size before (top), and after (bottom) electric stimulation of the nIII (20 s, 10 Hz). h EMG activity was recorded in the dorsal rectus (DR, top) as a control, whereas no responses were observed in the iris sphincter muscle (ISM, bottom) after a four-pulse electric stimulation of the oculomotor nerve (nIII; 10 Hz). i Dual tracer injections were performed to investigate putative connections between the iris and the brain. No retrogradely labeled ciliary ganglion cells were found, although abundant terminals were observed in the muscles innervated by the nIII, including the dorsal rectus (DR, right). j Preparation used to test the consensual PLR in lampreys (left). Normalized averaged data (mean ± SD, right) for pupil area in response to light to one eye. The blue line shows the pupil area for the unilluminated eye, and the black line for the illuminated eye (N = 2). The shaded areas in the graphs indicate error bands. Scale bar = 100 µm in i. n.s. not significant.
In other vertebrates, the fibers from the EWN exit the brain through the nIII1,23. Thus, we first compared pupil contraction before and after sectioning the nIII bilaterally, but no significant PLR reduction was observed (Fig. 2b, c; paired t-test p = 1 for both). We also performed electrical stimulation of the nIII aimed to evoke pupil contraction (N = 3). However, neither long stimulation trains with short pulses (30 s stimulation, 10 ms duration pulses, 10 Hz), nor a single long pulse stimulation (one pulse of 10 s duration) resulted in changes in pupil diameter or EMG activity in the iris, despite the activation of the extraocular muscles24 (Fig. 2g, h). In other vertebrates, the nIII targets the ciliary ganglion, which in turn projects to the iris sphincter muscle1,23. We applied neurobiotin to the nIII to anterogradely label putative terminals innervating the ciliary ganglion, and dextran-rhodamine in the iris to retrogradely label the ciliary ganglion cells (Fig. 2i left). Sagittal sections of the eyes together with their surrounding tissues were analyzed but no ganglionic neurons could be found, although clear anterogradely labeled terminals could be observed targeting the muscles innervated by the nIII24 (Fig. 2i right). However, no terminals were found in other areas. Additionally, sagittal sections were made of the intact head, following the nIII from its exit in the brain to the eye, but no evidence of a ciliary ganglion was found, fully confirming its absence in lampreys (N = 2; Supplementary Fig. 1a). We also investigated the presence of a consensual PLR (N = 2; Fig. 2j left). However, only the illuminated eye underwent pupil constriction (Fig. 2j right), as previously reported8. These results indicate that the EWN-mediated mechanism known in other vertebrates is not present in lampreys and that lampreys lack a ciliary ganglion as previously suggested25. No reduced pupil contraction was observed after total removal of the central nervous system to isolate the eye (N = 7; Fig. 2b–f; paired t-test p = 1 in b, c, f, and p = 0.734 in e), fully confirming that the PLR observed in lampreys is intrinsic to the eye.
The isolated iris shows PLR mediated by melanopsin
In other vertebrates, a mechanism intrinsic to the iris has been reported, in which the muscle fibers express melanopsin and act as photoreceptors contracting the pupil directly proportional to the amount of light3,4,5. We investigated this in lampreys, isolating the iris and applying light (N = 37; Fig. 3a), which resulted in a clear PLR (Fig. 3b), suggesting that, as in other vertebrates, muscle fibers express melanopsin and mediate pupil reduction. Since it is difficult to isolate the fragile iris without affecting its integrity, small parts of the retina remained in some cases. Thus, we isolated the iris in the presence of atropine (1 mM), an antagonist of muscarinic ACh receptors, to block cholinergic transmission and prevent any possible influence of the retina (see below; Fig. 3c–f; Supplementary Movie 2). Reduction rates were in general larger than those observed in intact eyes, most likely because the iris sphincter muscle must overcome less resistance and can thus achieve a larger contraction. The iris-intrinsic PLR is mediated by melanopsin in other vertebrates4,6,26, which has been shown to be expressed in the fibers of the iris sphincter muscle5. Although melanopsin expression was previously reported in the lamprey retina27, its presence in the iris was unknown. However, the presence of an iris-intrinsic PLR in lampreys, and previous experiments showing that the lamprey PLR achieves its maximum contraction within the absorption wavelength range of melanopsin8, suggest that melanopsin also mediates the lamprey iris-intrinsic PLR. Thus, we analyzed the PLR in the isolated iris after blocking melanopsin with AA92593 (60 µM), a selective and reversible antagonist of melanopsin-mediated phototransduction (N = 10). Light stimulation resulted in clear contraction (Fig. 3d, black line) that was significantly reduced after the application of the melanopsin antagonist (Fig. 3d, magenta line; paired t-test p < 0.001). These effects were reverted after the washout of the melanopsin antagonist (Fig. 3d, green line; paired t-test p = 1). These results indicate that, as in mice5, melanopsin is expressed in the muscle fibers of the iris sphincter muscle and mediates a PLR. Our results show that this mechanism was already present before lamprey divergence and was maintained in several vertebrate groups whereas in others, including humans, has been lost4,28.
a Experimental setup to analyze pupil contraction in the isolated iris, submerged in artificial cerebrospinal fluid (aCSF). b Graph showing a representative normalized pupil area through time in response to light stimulation to the isolated iris. c Experimental setup to evaluate the role of melanopsin in the isolated iris. aCSF with atropine to block any potential contribution of the retinal cholinergic pathway was used as a control, and AA92593 was used to block melanopsin. d Graph showing the normalized pupil area through time in response to light stimulation to the isolated iris in control conditions (black line), after applying the melanopsin antagonist AA92593 (magenta line), and after washout (green line; paired t-test p < 0.001). e Experimental setup to pharmacologically test the contribution of gap junctions to the PLR in the isolated iris. aCSF with atropine to block any potential contribution of the retinal cholinergic pathway was used as a control, and octanol was used to block gap junctions. f Graph showing the normalized pupil area through time in response to light stimulation to the isolated iris in control conditions (black line), after applying the gap junction blocker octanol (yellow line, paired t-test p < 0.001), and after washout (green line). g Transmission electron microscopy images showing the presence of gap junctions between adjacent muscle fibers of the iris sphincter muscle. Scale bars = 1 µm in g left and 0.5 µm in g right.
Gap junctions contribute to the contraction of the iris sphincter muscle
In mice, gap junctions between adjacent muscle fibers in the iris transfer changes in excitability in response to light to other muscle fibers5. To test whether gap junctions play a role in the lamprey PLR, we blocked them pharmacologically in the isolated iris using octanol (N = 3; Fig. 3e; 0.5 mM) as previously reported in mice5. Again, atropine was used to block any possible contribution of the ACh retinal mechanism described below. As shown in the representative example of Fig. 3f, pupil contraction (black line) was significantly reduced after blocking gap junctions (yellow line; paired t-test p < 0.001), indicating that these intercellular channels participate in the transmission of the excitability changes triggered by melanopsin. The effects exerted by the gap junction blocker were reverted after washout (Fig. 3f, green line; paired t-test p = 1). The presence of gap junctions in the fibers of the iris sphincter muscle was confirmed by using transmission electron microscopy (N = 3; Fig. 3g). These results show that the participation of gap junctions in the iris sphincter muscle contraction was already present in early vertebrates, and further reinforce that the iris-intrinsic PLR mechanism described in mice was already present at the base of vertebrate evolution.
Retinal cells provide cholinergic innervation of the iris
In mice, it has been suggested that ipRGCs can evoke pupil contraction via direct cholinergic projections to the iris2,7. Although this mechanism has only been suggested in mice, we decided to test this possibility in the lamprey. We first isolated the iris and applied ACh (100 µM; N = 9), which resulted in a clear pupil contraction (Fig. 4a; Supplementary Movie 3). Given the absence of a ciliary ganglion and any brain-mediated PLR (see above), these results indicate that there may be an ACh release in the iris, likely via axons originating within the eye. To confirm the presence of an eye-intrinsic mechanism mediated by ACh, we isolated the eye and analyzed the PLR before and after the application of atropine (N = 5; Fig. 4b; 1 mM). As shown in Fig. 4b (left), the PLR (black line) was significantly reduced (paired t-test p < 0.001) when the cholinergic transmission was blocked (orange line). These results suggested that an axonal component innervating the iris sphincter muscle is present in lampreys. To investigate the presence of axons in the iris, we immunostained for acetylated tubulin, and observed axons coursing from the retinal region to the iris both in the flat-mounted eye (Fig. 4c and Supplementary Fig. 1b) and in sagittal sections (Fig. 4d). We next aimed to uncover the origin of these projections. For this, we first applied neurobiotin in the iris and found retrogradely labeled neurons in the retina (N = 11). Retrogradely labeled cells were evenly distributed throughout the retina, and no obvious regionalization was observed. Neurobiotin-labeled cells were found isolated (Supplementary Fig. 1c), but in some cases clusters of numerous retrogradely labeled cells were observed, both in close apposition (Fig. 4e) and forming groups of more separated cells (Fig. 4g). The low molecular weight of neurobiotin (287 Da), allows this tracer to cross gap junctions29, which are known to be present in the lamprey retina30,31. Thus, we hypothesized that some of the labeled cells were not retrogradely labeled, but rather electrically coupled to cells projecting to the iris. To confirm this, we did injections both combining neurobiotin and dextran-rhodamine (N = 2; Fig. 4f) and only with dextran-rhodamine (3000 Da; N = 11; Fig. 4h, i). The high molecular weight of dextran-rhodamine does not allow this molecule to cross gap junctions and, accordingly, no dextran-rhodamine retrogradely labeled cell clusters as those observed with neurobiotin were found, thus indicating that cells projecting to the iris are electrically coupled to other cells in the retina. As for neurobiotin application, no obvious lateral regionalization was found, and iris-projecting cells (retrogradely labeled with dextran-rhodamine) were evenly distributed throughout the retina. In Fig. 4f, a representative example is shown of a retrogradely labeled cell, both with neurobiotin and dextran-rhodamine. Iris-projecting neurons were more abundant in the inner nuclear layer (INL) region proximal to the inner plexiform layer (IPL; Fig. 4f, i; see 31–34 for lamprey retinal organization). In the INL, iris-projecting cells were also found in the layer of horizontal cells (Fig. 4i, blue arrowhead), and, occasionally, iris-projecting cells were also present in the IPL (not shown). Cells projecting from the retina were always found in the IPL and INL. The same applied to those cells labeled with neurobiotin (including both iris projecting and electrically coupled cells), indicating that the cells electrically coupled with those projecting to the iris are also located in these two layers. Interestingly, the clusters of neurobiotin-labeled cells in close apposition were always found in the INL, in the layer of inner horizontal cells (Fig. 4e).
a Experimental setup to analyze pupil contraction in the isolated iris, submerged in aCSF (left), and a representative normalized pupil area in response to acetylcholine (ACh) application (right). b Experimental setup (left) to analyze the contribution of ACh to the PLR in the isolated eye, using the cholinergic antagonist atropine, both bath-applied and injected in the eye. Normalized averaged data (mean ± SD, right) for pupil area in response to light stimulation in control conditions (black line), and after atropine application (orange line); p < 0.001 (paired t-test). c Photomicrograph showing the expression of acetylated tubulin in a flat-mounted eye. Labeled axons are coursing from the retina (blue arrowheads) to the iris (white arrowheads). The location of the image can be seen in the schematic below. See also Supplementary Fig. 1b. d Sagittal section of the eye showing acetylated tubulin labeling in the retina, and axons towards the iris (white arrowheads). e Neurobiotin-labeled cluster of neurons located in the INL, in the layer of horizontal cells. See also Supplementary Fig. 1c. f Cell labeled both with neurobiotin and dextran-rhodamine. On the left, the labeling for each of the tracers is shown. g Neurobiotin-labeled cells in a flat-mounted retina. h Representative injection site of dextran-rhodamine in the iris and retrogradely labeled cells can be seen in the retina (white arrowhead). i Dextran-rhodamine labeled cells in the inner nuclear layer (INL) of the retina, both proximal to the inner plexiform layer (IPL; white arrowheads) and in the layer of horizontal cells (blue arrowhead). j Representative dextran-rhodamine retrogradely labeled neuron in the INL region proximal to the IPL (left) which is also acetylcholinesterase (AChE) positive (middle, see arrowheads). On the right, magnification of both. k Representative dextran-rhodamine retrogradely labeled (left) and AChE positive (right) neurons are also present in INL, but in the layer of horizontal cells. Scale bars = 100 µm in c, d, e, and h; 50 µm in f; 10 µm in the f insets; 20 µm in g and i; 25 µm in j and k. SL segment layer, ONL outer nuclear layer, OPL outer plexiform layer.
Our pharmacological experiments indicate that the retinal cells that project to the iris are cholinergic (see above) and, in agreement with this, cholinergic neurons in lampreys were reported in the INL and the IPL32, where iris-projecting cells are located. To further confirm that the retinal cells that project to the iris are cholinergic, we carried out experiments combining dextran-rhodamine application in the iris with immunohistochemistry for choline acetyltransferase (ChAT). However, we tried several antibodies that did not result in clear labeling, and the antibody previously used in lampreys is not available anymore32. Thus, we used an alternative approach by carrying out acetylcholinesterase (AChE) histochemistry to detect cholinergic neurons, combined with dextran-rhodamine injections to label iris-projecting cells (N = 6, n = 8). AChE expression was observed in the same INL and IPL locations where ChAT immunoreactivity had been previously reported (Fig. 4j)32, indicating that, as in mammals, AChE is a good cholinergic secondary marker in the retina33. As expected, numerous retrogradely labeled cells were found to express AChE both in the INL region proximal to the inner plexiform layer (Fig. 4j), and in the INL region of horizontal cells (Fig. 4k). Remarkably, melanopsin expression in the lamprey retina was also reported both in the INL and the IPL27,34,35. Nearly all cells in the INL layer of horizontal cells express melanopsin27,34, indicating that both the cell clusters of electrically coupled cells and those projecting to the iris express melanopsin in this region. Additionally, melanopsin expression was also reported in the INL proximal region to the IPL, and in the IPL itself27,34. The presence of melanopsin in the same regions where iris-projecting cells are located suggests that iris-projecting cells likely express melanopsin, although additional experiments are necessary to confirm this. Regarding ACh, choline acetyltransferase immunoreactivity was shown in both the IPL and the INL32. In the INL, most cholinergic cells were reported in the layer of cells proximal to the IPL, in the same location where iris-projecting cells are more abundant, in agreement with our pharmacological results and our AChE histochemistry experiments combined with dextran-rhodamine application showing that iris-projecting cells are cholinergic. Altogether, these results indicate that lampreys have direct cholinergic projections from the retina to the iris that can evoke pupil contraction. Considering previous results reporting melanopsin expression in lampreys27,34, and that the PLR in these animals has been suggested to be mediated only by melanopsin8, cholinergic cells may also express melanopsin and be electrically coupled via gap junctions to other melanopsin-expressing cells.
The iridal and retinal PLR mechanisms complement each other
The analysis of the PLR at a 15 Hz framerate allowed us to uncover the details of pupil contraction dynamics. We noticed that, in general, pupil size reduction was not constant, but in many eye-intact experiments a clear decrease in the reduction rate occurred after a few seconds. To confirm this decrease in pupil reduction, we analyzed the presence of significant slope changes (see Methods). In the graph of Fig. 5a, the significant slope changes are shown for the averaged data of 7 intact eyes. At ⁓17.6 s, the speed of pupil reduction decreased, and around 33.2 s increased again. Interestingly, in this time window, pupil contraction not only slowed down but reverted giving rise to pupil dilation when light stimulation was presented to the isolated iris (Fig. 5b). This shows that the iris-intrinsic mechanism alone has some limitations in evoking a continuous pupil reduction, but this limitation is counteracted by the cholinergic projections from the retina.
a Graph showing the normalized pupil area average data of 7 animals (47 experiments) through time in response to light stimulation to the intact eye (black line), indicating the significant slope change points with blue vertical lines. The red line shows the fitted data. The red vertical line indicates the onset of muscle contraction. b Graph showing the normalized pupil area through time in response to light stimulation to the isolated iris, indicating the significant slope change points with blue vertical lines. The black line shows the average data of 5 animals, whereas the red line shows the fitted data. c Summarizing schematic. Muscle fibers in the iris sphincter muscle express melanopsin evoking an iris-intrinsic PLR. Additionally, cholinergic neurons project from the retina to the iris, mediating a second eye-intrinsic PLR mechanism. These cells potentially express melanopsin and/or are electrically coupled to melanopsin-expressing cells.
Discussion
Our results show that the PLR in lampreys is mediated by two mechanisms intrinsic to the eye, one iris-intrinsic mechanism mediated by melanopsin, and the other mediated by cholinergic projections from the retina (Fig. 5c). However, a brain-controlled PLR is absent. The lack of a brain-mediated mechanism explains the absence of a consensual PLR (Fig. 2j), which is a consequence of the bilateral innervation of the EWN from pretectum1. Additionally, the temporal dynamics of the PLR melanopsin component in other vertebrates explain the slow nature of the lamprey PLR36. In other vertebrates, the iris-mediated mechanism allows a sustained pupil contraction under strong steady light, which is not possible to achieve via the brain-mediated mechanism due to the small amount of light that reaches the retina through the pupil. In this manner, the highly photosensitive retinas of nocturnal/crepuscular animals are protected4. At least two spectra types of photoreceptors, short and long wavelength sensitive, are present in the lamprey retina, and their response properties to light indicate that they are homologous to rods and cones, respectively37,38,39. Lampreys can live in deep waters, suggesting that they also have highly photosensitive eyes40. However, our results show that the iris-intrinsic mechanism alone is not sufficient to evoke a reliable PLR in these animals. Thus, a second mechanism, also mediated by melanopsin, evokes the PLR via cholinergic projections from the retina that, synergistically with the iris intrinsic mechanism, allows a consistent PLR. This is surprising given that both the iris-intrinsic and the retina-controlled mechanisms are mediated by melanopsin. However, it has been shown in mice that, although retinal and iridal melanopsin share a common phospholipase C-mediated phototransduction pathway, the downstream mechanisms are different4. It is likely that a similar situation is present in lampreys so that the iris and the retina-mediated mechanisms have different phototransduction dynamics, despite their common melanopsin activation, and therefore they complement each other allowing a robust and continuous pupil contraction. The pupil contraction evoked by the lamprey PLR is not very extensive when compared to other vertebrates1. Unfortunately, the behavioral data in predatory lampreys is very limited and it is therefore difficult to speculate about the role of their PLR. However, it would be unlikely that a PLR in lampreys based on two different mechanisms would be maintained unless it has some functional relevance, although additional experiments are needed to test its actual contribution.
The presence of direct retino-iridal projecting cells raises the question of what retinal cell type they belong to. Although dextran-rhodamine tracer injections allowed the observation of clear retrogradely labeled cell bodies, this type of labeling did not allow a clear observation of the neuronal processes, and therefore morphological reconstructions were not feasible. However, no positive fibers were observed in the optic nerve after dextran-rhodamine injections, as one would expect if retrogradely labeled cells were ganglion cells. Additionally, it is known that cholinergic cells in lampreys correspond to amacrine cells32,34. Thus, it is likely that the cells that project to the iris are amacrine cells, although additional experiments are needed to confirm this.
The lack of a brain-mediated mechanism is surprising given the high degree of conservation of the visual areas in the brain. The pretectum, which mediates the PLR in other vertebrates, shows conserved features from lampreys to mammals, including the same role mediating the optokinetic reflex9. Our results suggest that pretectal-mediated gaze stabilizing responses appeared earlier in vertebrate evolution than the pretectal-mediated PLR and that the protection of the retina from light was initially based on eye-intrinsic mechanisms. Although the retina-mediated mechanism has been suggested in mice2,7, its contribution to the PLR is still controversial5, and, to our knowledge, our study is the first that demonstrates a direct projection from the retina to the iris. The lack of data in other vertebrates hinders a direct homology between the lamprey mechanism described here and the one proposed in mice. However, our results suggest that an iris-intrinsic PLR and one mediated by direct projections from the retina were present in early vertebrates and were conserved in some vertebrate groups, whereas the brain-mediated PLR appeared later in evolution.
Methods
Animals
Experiments were performed in 63 lampreys: 25 adult and 18 postmetamorphic sea lampreys (Petromyzon marinus), and 20 adult river lampreys (Lampetra fluviatilis). Animals were kept in tanks enriched with sand and/or stones, continuously oxygenated and filtrated, in a light-controlled room (12 light/12 darkness) with a light intensity between 120-280 lux emitted by white, fluorescent lamps (Mazda Fluor Lumiere du jour 36 W). We have complied with all relevant ethical regulations for animal use, to this end, and all procedures were approved by the Xunta de Galicia under the supervision of the University of Vigo Committee for Animal Use in Laboratory in accordance with the directive 2010/63/EU of the European Parliament and the RD 53/2013 Spanish regulation on the protection of animals used for scientific purposes. Minimizing the suffering and reducing the number of lampreys employed while maximizing the obtained data was a priority when designing the experiments.
Experimental preparations
To investigate the brain contribution to the PLR, an isolated preparation of the brain with the eyes was used, so that electrophysiological experiments could be combined with pupil tracking and acute lesions or stimulation. For this, animals were deeply anesthetized prior to all dissections with tricaine methane sulfonate (MS-222; 100 mg/L; Sigma-Aldrich). Decapitation was performed between the third and fourth gills and the preparation was immediately immersed in a chamber containing refrigerated artificial cerebrospinal fluid (aCSF) with the following composition (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 25 NaHCO3, saturated with 95% O2/5% CO2 (vol/vol). All the muscles, viscera, and skin were removed, exposing the eyes and the brain. Finally, the preparation was placed in a transparent chamber perfused with refrigerated aCSF. In this preparation, lesions were performed to analyze the encephalic contribution to the PLR. To analyze the presence of a putative Edinger-Westphal nucleus (EWN) the oculomotor nerves were cut and, to discard any possible encephalic contributions, the brain was totally removed. To investigate the presence of an eye/iris-intrinsic PLR, the eyes were dissected out from the preparation described above and placed in a transparent chamber perfused with ice-cooled aCSF. The iris was isolated by pinning down the eyes and cutting it out using small Castroviejo scissors. Then, the isolated iris was pinned down in a transparent chamber perfused with cold aCSF.
Pupil tracking
The videos were recorded using a digital camera USB-N&B NIR (IDS) coupled to a vari‐focal manual iris lens with the infrared detector, model T3Z2910CS‐IR (Computar, CBC Group). All the videos were recorded in darkness, with the only constant source of light being an IR illuminator, model CM-IR56 (CMVision). Experiments were carried out during the day, in the morning hours. To evoke pupil contraction, a white LED was used to apply light stimulation presented to one eye. First, in the eye-brain preparation, the isolated eye or iris was left in darkness for 20 min, and then light stimulation was applied for 5 min. These parameters were chosen to minimize the duration of the experiment while ensuring reliable pupil contraction and the viability of the preparation.
Iris contraction was tracked using DeepLabCut22, a Python-based open-source software package to estimate poses through artificial neural networks. To achieve this, 20 keyframes of each recorded video were selected randomly and four labels were placed at the external edge of the iris in each frame (Top, Right, Bottom, and Left). Then, the neural network was trained and evaluated. When poor performance of the trained network was observed, based on its evaluation and/or visual inspection of the analyzed labeled videos, refinements were performed, and the network was further retrained. Subsequently, the trained network was used to extract the position of the labels throughout the videos.
Electrophysiological recordings
Tungsten microelectrodes (~1–5 MΩ) connected to a differential AC amplifier (model 1700, A-M systems) were employed to perform extracellular recordings of muscle and/or neuronal activity. Signals were digitized at 20 kHz using pClamp 10.4 software. Electrical stimulation was performed using borosilicate micropipettes (od = 1.5 mm, id = 1.17 mm; Hilgenberg) filled with aCSF connected to a stimulus isolation unit (MI401, Zoological Institute, University of Cologne). Microelectrodes and micropipettes were placed in the areas of interest employing micromanipulators (model M-3333, Narishige). To test the involvement of a putative EWN, electric stimulation of the oculomotor nerve was performed while video-recording pupil contraction and/or recording EMG activity in the iris. EMG activity in the dorsal rectus was used as a control (Fig. 2h). In some cases, an incision was done in the iris to ensure that the recording electrode was in contact with the sphincter muscle. The stimulation intensity was tested from 0.01 to 1 mA, and both long stimulation trains with short pulses (30 s stimulation, 10 ms duration pulses, 10 Hz) and single pulse stimulations with long duration (10 s) were tested. To ensure that the lack of responses was not due to the invalidity of the preparation, extracellular recordings in reticulospinal neurons in response to optic tract stimulation were carried out as a control.
Drug applications
The role of melanopsin mediating the iris-intrinsic PLR was tested using AA92593 (60 µM; MedChemExpress), a selective and reversible antagonist of melanopsin-mediated phototransduction without affecting rod- and cone-mediated responses. AA92593 was bath-applied to the iris, isolated as previously described. To assess cholinergic innervation to the iris, acetylcholine (ACh) 100 µM (Sigma-Aldrich) was bath-applied to the isolated iris while monitoring pupil contraction. A fragment of extraocular muscle was also used as a control to test ACh activation. Atropine (1 mM; Sigma-Aldrich) was employed to block cholinergic transmission both bath-applied to the isolated iris and to isolated eyes. In the latter case, bath-applied administration was combined with atropine injections in the eye to ensure the exposure of the iris muscle to the drug. To analyze the existence of gap-junction between iris muscle fibers, octanol (500 µM; Carlo Erba) was used as a reversible blocker5. Experiments were performed in the isolated iris. In all cases, drug effects were reversed (partially or totally) washing out with clean aCSF.
Anatomical tract tracing
Neurobiotin (Vector Laboratories) or dextran-rhodamine (tetramethylrhodamine, 3000 Da, Invitrogen, D3308) was used to study the neuronal circuits involved in the PLR. Tracer crystals were applied in the cut oculomotor nerve, and/or the iris. For tracer application in the iris, either a lesion with a sharp needle or an opening using Castroviejo scissors was made to ensure the direct application to the muscle. After the tracer applications, the samples were thoroughly washed and submerged in aCSF at 4 °C for 1 to 4 days in darkness to allow the transport of the tracer. Then, the preparations were fixed in 4% formaldehyde and 14% saturated picric acid in 0.1 M phosphate buffer (PBS), pH 7.4, for 12–24 h, and cryoprotected in 20% (wt/vol) sucrose in PBS for 4–12 h. Afterward, they were embedded in an OCT compound (Tissue-Tek, Sakura), cut in transverse sections of 30 μm thickness in a cryostat (Leica cm1950), and collected on gelatinized slides. To detect neurobiotin, sections were incubated with Cy2-conjugated streptavidin (Alexa Fluor 488, 10,000 Da, Invitrogen, D22910) 1:1000 in blocking solution (1% bovine serum albumin, 0.1% sodium azide and 0.3% Triton X-100 in PBS). The sections were subsequently mounted in DAPI-containing glycerol (DAPI Fluoromount-G, SouthernBiotech). For some injections, neurobiotin was detected using the ABC kit (SIGMA), and the labeling was observed flat-mounting fragments of the retina. For this, retina fragments were fixed in 1% glutaraldehyde, 4% paraformaldehyde, and 15% saturated picric acid in PB for 24 h. Samples were placed over a slide and covered with melted agar 4%. Once agar solidified, the samples were immersed again in melted agar a 4% and horizontally sectioned in a vibratome. 100–200 µm thick sections were obtained and subsequently incubated in 1% sodium borohydride in PBS for 1 h, in 15% H2O2 in PBS for 1 h, and 1% bovine serum albumin, 0.3% Triton X-100 in PBS for 1 h. Sections were then incubated in an ABC kit, diluted 1/100 in PBS for 1 h, washed in PBS three times, and developed with 0.5% of 3,3’-diaminobenzidine (SIGMA) and 0.01% H2O2 in PB for about 15 min. Sections were mounted with glycerol for observation.
Hematoxylin-eosin and Gömöry’s trichrome staining
Eyes were fixed in buffered 4% paraformaldehyde and 15% saturated picric acid, and paraffin embedded following a standard protocol. 8 µm thick sections were deparaffined, hydrated, and stained with Mayer hematoxylin and eosin Y (0.2%), or with Weigert hematoxylin and a trichromic solution containing cromo2R, light green, and phosphotungstic acid. After the staining, sections were dehydrated, cleared, and coverslipped with Eukitt (ORSAtec). Hematoxylin-eosin staining was also performed in cryostat sections, obtained as described above.
Electron microscopy
Adult and postmetamorphic lamprey eyes were dissected out and fixed in 1% glutaraldehyde, 4% paraformaldehyde, and 15% saturated picric acid in PB for 24 h. They were osmified in 1% osmium tetroxide in PB for 30 min, dehydrated in ethanol, and embedded in Durcupam (Fluka). Ultrathin sections were obtained in an ultramicrotome (Reitcher), placed onto copper grids, and contrasted with uranyl acetate and lead citrate. Before cutting ultrathin sections, some semithin sections (0.5 µm) were obtained, placed onto slices, stained with toluidin blue, and observed and photographed at light microscopy.
Immunofluorescence
Eyes from postmetamorphic and adult lampreys were fixed in 4% paraformaldehyde in PB for 24 hours. After that, the caudal half of the ocular globe and the eye lens were removed, trying to keep the lens covering tissue. Some meridian cuts were done in the remaining ocular globe to get extended and nearly flat samples. These samples were then incubated in 1% sodium borohydride in PBS for 1 h, and 1% bovine serum albumin, and 0.3% Triton X-100 in PBS for 1 h. After that, they were immersed in a monoclonal anti-mouse acetylated tubulin (SIGMA, Clon: 6-11B-1, ascites fluid. Code: T-6793; Lot 054K4835) diluted 1/1000 in PBS for 16-18 h. Samples were washed several times in PBS and incubated in a goat anti-mouse conjugated with Alexa fluor 488 (Invitrogen) diluted 1/100 in PBS for 1 h. After several washes in PBS, samples were placed and extended onto slides, coverslipped with anti-Fade reagent (Molecular probes), and observed and photographed at fluorescence microscopy.
Acetylcholinesterase histochemistry
To confirm that direct projections from the retina to the iris are cholinergic, we combined dextran-rhodamine application in the iris to detect retinal cells projecting to the iris (see above) with acetylcholinesterase histochemistry (method of Karnovsky and Roots41) to detect cholinergic somata. After tracer transport, the eyes were fixed in 10% formol and calcium chloride 0.09 M for 1–6 h and cryoprotected in 20% (wt/vol) sucrose in PBS for 4–12 h. 30 μm transverse sections of the eyes were obtained in a cryostat on gelatinized slides and then washed in maleic acid buffer pH 6.5. Afterward, sections were incubated in a solution with the following composition (in mM): 2 acethylcholine iodides, 10 sodium citrate, 3 copper sulfate, and 0.5 potassium ferrocyanide in maleic acid pH 6.5 at 26 °C for 30 min-2h. After washing in maleic acid buffer, sections were mounted as described above.
Image analysis
Photomicrographs were taken with a digital camera (Nikon DS-Ri2) coupled to a Nikon ECLIPSE Ni-E fluorescence microscope. Confocal images were obtained with a Leica Stellaris 8 microscope 510. Images were processed using ImageJ 1.53k and GIMP 2.1. Images were only adjusted for brightness and contrast. Figures were made using Adobe Illustrator CC 2019.
Quantification
Data analysis was performed using custom-written functions in Matlab R2020b. To calculate pupil contraction, videos were first analyzed to extract the positions of the four labels above described using DeepLabCut. Then, the distances between the labels in the top and bottom edges of the pupil, and the right and left edges were used to calculate the radius in the X and Y axes of the pupil, respectively. This was in turn used to calculate the area of the pupil applying the formula for an ellipse. Area data were normalized to the first frame of light stimulation or drug application (for ACh application) to calculate the percentage of reduction. To detect significant slope changes, we used the Matlab function findchangepts, which returns the index at which the mean of x changes most significantly.
Statistics and reproducibility
Statistical analysis was done using JASP 0.16. Paired t-tests were used to compare pupil reduction between different conditions. Throughout the Figures, sample statistics are expressed as means ± SD. While data was pooled for several recordings per animal, each variable was also tested for several preparations. These are indicated as n for the number of experiments performed, and N for animal numbers. The number of animals and replications were as follows. The experiments to determine whether the brain contributes to the PLR (Fig. 2b–f) were performed on seven animals. Three repetitions were analyzed per condition. The experiments to detect the presence of a consensual PLR (Fig. 2j) were performed on two animals, and three replications were analyzed. To analyze the PLR in the isolated iris, 37 animals were used. Of these, in 10 animals the melanopsin antagonist was also tested, and the effect of the octanol was analyzed in three animals (Figs. 3a–f and 4a, b). In these experiments, no replications were performed. Electrophysiological experiments (Fig. 2h) were performed on three animals with three replications each. The anatomical experiments carried out to characterize the lamprey iris sphincter muscle (Fig. 1) were performed on 12 animals. Tracer injections to analyze retino-iridal projections (Fig. 4d–k) were performed in 11 animals and tracer injections combined with whole sectioning of the head to determine the presence of a ciliary ganglion in two animals (Fig. 2i, left). Finally, the experiments to detect cholinergic neurons through AChE histochemistry (Fig. 4j–k) were performed on eight eyes from six animals. The degree of statistical significance is indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data are provided with this paper and can be downloaded at the following link (https://doi.org/10.5281/zenodo.12799661)42. Further information and requests should be addressed to the corresponding author.
References
Douglas, R. H. The pupillary light responses of animals; a review of their distribution, dynamics, mechanisms and functions. Prog. Retin. Eye Res. 66, 17–48 (2018).
Vugler, A. et al. A role for the outer retina in development of the intrinsic pupillary light reflex in mice. Neuroscience 286, 60–78 (2015).
Provencio, I., Jiang, G., De Grip, W. J., Hayes, W. P. & Rollag, M. D. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl Acad. Sci. USA 95, 340–345 (1998).
Xue, T. et al. Melanopsin signaling in mammalian iris and retina. Nature 479, 67–73 (2011).
Wang, Q. et al. Synergistic signaling by light and acetylcholine in mouse iris sphincter muscle. Curr. Biol. 27, 1791–1800.e5 (2017).
Cheng, S. et al. Melanopsin mRNA in the iris of red-eared slider turtles (Trachemys scripta elegans). J. Herpetol. 51, 538–551 (2017).
Semo, M., Gias, C., Ahmado, A. & Vugler, A. A role for the ciliary marginal zone in the melanopsin-dependent intrinsic pupillary light reflex. Exp. Eye Res. 119, 8–18 (2014).
Morshedian, A., Huynh, T. H., Frederiksen, R., Fain, G. L. & Sampath, A. P. Pupillary light reflex of lamprey Petromyzon marinus. Curr. Biol. 31, R65–R66 (2021).
Wibble, T., Pansell, T., Grillner, S. & Pérez-Fernández, J. Conserved subcortical processing in visuo-vestibular gaze control. Nat. Commun. 13, 4699–469 (2022).
Kardamakis, A. A., Saitoh, K. & Grillner, S. Tectal microcircuit generating visual selection commands on gaze-controlling neurons. Proc. Natl Acad. Sci. USA 112, 1956 (2015).
Kardamakis, A. A., Pérez-Fernández, J. & Grillner, S. Spatiotemporal interplay between multisensory excitation and recruited inhibition in the lamprey optic tectum. eLife 5, https://doi.org/10.7554/eLife.16472 (2016).
Suzuki, D. G., Pérez-Fernández, J., Wibble, T., Kardamakis, A. A. & Grillner, S. The role of the optic tectum for visually evoked orienting and evasive movements. Proc. Natl Acad. Sci. USA 116, 15272–15281 (2019).
Suryanarayana, S. M., Pérez-Fernández, J., Robertson, B. & Grillner, S. The evolutionary origin of visual and somatosensory representation in the vertebrate pallium. Nat. Ecol. Evol. 4, 639–651 (2020).
Barandela, M. et al. Unravelling the functional development of vertebrate pathways controlling gaze. Front. Cell. Dev. Biol. 11, 1298486 (2023).
Collin, S. P. & Pottert, I. C. The ocular morphology of the southern hemisphere lamprey Mordacia mordax Richardson with special reference to a single class of photoreceptor and a retinal tapetum. Brain Behav. Evol. 55, 120–138 (2000).
Collin, H. B., Ratcliffe, J. & Collin, S. P. The functional anatomy of the cornea of the Shorthead lamprey, Mordacia mordax (Mordaciidae, Agnatha): a comparison between downstream and upstream migrants. J. Morphol. 284, e21552 (2023).
Collin, H. B., Ratcliffe, J. & Collin, S. P. The functional anatomy of the cornea and anterior chamber in lampreys: insights from the pouched lamprey, Geotria australis (Geotriidae, Agnatha). Front. Neuroanat. 15, 786729 (2021).
Dickson, D. H. & Graves, D. A. in The Biology of Lampreys, (eds M. W. Hardisty, M. W. & Potter, I. C.) 43–94 (Academic Press, 1981).
Kleerekoper, H. in Biology of Lampreys (eds. Hardisty, M. W. & Potter, I. C.) 373–404 (Academic Press, 1972).
Herrera, A. M., Martinez, E. C. & Seow, C. Y. Electron microscopic study of actin polymerization in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, 1161 (2004).
Pérez-Fernández, J., Kardamakis, A. A., Suzuki, D. G., Robertson, B. & Grillner, S. Direct dopaminergic projections from the SNc modulate visuomotor transformation in the lamprey tectum. Neuron 96, 910–924.e5 (2017).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
McDougal, D. H. & Gamlin, P. D. Autonomic control of the eye. Compr. Physiol. 5, 439–473 (2015).
Fritzsch, B., Sonntag, R., Dubuc, R., Ohta, Y. & Grillner, S. Organization of the six motor nuclei innervating the ocular muscles in lamprey. J. Comp. Neurol. 294, 491–506 (1990).
Suzuki, D. G. et al. Comparative morphology and development of extra-ocular muscles in the lamprey and gnathostomes reveal the ancestral state and developmental patterns of the vertebrate head. Zool. Lett. 2, 10–13 (2016).
Xue, T. et al. Melanopsin signalling in mammalian iris and retina. Nature 479, 67–73 (2011).
Sun, L. et al. Distribution of mammalian-like melanopsin in cyclostome retinas exhibiting a different extent of visual functions. PLoS ONE 9, e108209 (2014).
Bouffard, M. A. The pupil. Continuum 25, 1194–1214 (2019).
Song, J., Ampatzis, K., Björnfors, E. R. & El Manira, A. Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature 529, 399–402 (2016).
Tonosaki, A., Washioka, H., Hara, M., Ishikawa, M. & Watanabe, H. Gap junctions and synaptic relations of horizontal cells in lamprey retina. Neurosci. Res. Suppl. 6, 107 (1987).
Fritzsch, B. & Collin, S. P. Dendritic distribution of two populations of ganglion cells and the retinopetal fibers in the retina of the silver lamprey (Ichthyomyzon unicuspis). Vis. Neurosci. 4, 533–545 (1990).
Pombal, M. A., Abalo, X. M., Rodicio, M. C., Anadón, R. & González, A. Choline acetyltransferase-immunoreactive neurons in the retina of adult and developing lampreys. Brain Res. 993, 154–163 (2003).
Criswell, M. H. & Brandon, C. Acetylcholinesterase and choline acetyltransferase localization patterns do correspond in cat and rat retinas. Vis. Res. 33, 1747–1753 (1993).
Wang, J. et al. Molecular characterization of the sea lamprey retina illuminates the evolutionary origin of retinal cell types. bioRxiv 2023.12.10.571000 (2023).
Jones, M. R., Grillner, S. & Robertson, B. Selective projection patterns from subtypes of retinal ganglion cells to tectum and pretectum: distribution and relation to behavior. J. Comp. Neurol. 517, 257–275 (2009).
Joyce, D. S., Feigl, B., Cao, D. & Zele, A. J. Temporal characteristics of melanopsin inputs to the human pupil light reflex. Vis. Res. 107, 58–66 (2015).
Suzuki, D. G. & Grillner, S. The stepwise development of the lamprey visual system and its evolutionary implications. Biol. Rev. Camb. Philos. Soc. 93, 1461–1477 (2018).
Morshedian, A. & Fain, G. L. Light adaptation and the evolution of vertebrate photoreceptors. J. Physiol. 595, 4947–4960 (2017).
Asteriti, S., Grillner, S. & Cangiano, L. A Cambrian origin for vertebrate rods. eLife 4, e07166 (2015).
Haedrich, R. L. A sea lamprey from the deep ocean. Copeia 1977, 767–768 (1977).
Karnovsky, M. J. & Roots, L. A “Direct-Coloring” thiocholine method for cholinesterases. J. Histochem. Cytochem. 12, 219–221 (1964).
Jiménez-López, C. et al. Source data for: Direct retino-iridal projections and intrinsic iris contraction mediate the pupillary light reflex in early vertebrates (Communications Biology) [Data set]. Zenodo https://doi.org/10.5281/zenodo.12799661 (2024).
Acknowledgements
We are grateful to Sten Grillner and Manuel A. Pombal for their constant support and valuable comments on the manuscript, to Daichi G. Suzuki for technical assistance and valuable comments, to Brita Robertson for her valuable comments on the manuscript, to Martiño Barreiro and Emma Rodríguez for technical support, to Eduardo Pena for providing hardware, and to Tobias Wibble and the river station of A Freixa for helping with lamprey supply. This work was supported by Proyectos I + D + i PID2020-113646GA-I00 funded by MCIN/AEI/ 10.13039/501100011033 and by “ERDF A way of making Europe”, the Ramón y Cajal grant RYC2018-024053-I funded by MCIN/AEI/ 10.13039/501100011033 and by “ESF Investing in your Future”, Xunta de Galicia (ED431B 2021/04 to JPF and ED481A 2022/433 to CNG- Programa de axudas á etapa predoutoral da Xunta de Galicia (Consellería de Cultura, Educación, Formación Profesional e Universidades)), and CINBIO.
Author information
Authors and Affiliations
Contributions
Conceptualization: C.J.L., J.P.F.; Experimental design: C.J.L., P.R.R., M.M., J.P.F.; Data acquisition: C.J.L., P.R.R., M.B., C.N.G., M.M.; Data analysis: C.J.L., P.R.R., M.B., C.N.G., M.M., J.P.F.; Writing: C.J.L., M.M., and J.P.F. with inputs from all the authors; Supervision and funding acquisition: J.P.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Tom Baden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Benjamin Bessieres. [A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiménez-López, C., Rivas-Ramírez, P., Barandela, M. et al. Direct retino-iridal projections and intrinsic iris contraction mediate the pupillary light reflex in early vertebrates. Commun Biol 7, 993 (2024). https://doi.org/10.1038/s42003-024-06699-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06699-0
- Springer Nature Limited