Abstract
Following evidence of waning immunity against both infection and severe disease after 2 doses of the BNT162b2 vaccine, Israel began administering a 3rd BNT162b2 dose (booster) in July 2021. Recent studies showed that the 3rd dose provides a much lower protection against infection with the Omicron variant compared to the Delta variant and that this protection wanes quickly. However, there is little evidence regarding the protection of the 3rd dose against Omicron (BA.1/BA.2) severe disease. In this study, we estimate the preservation of immunity from severe disease up to 7 months after receiving the booster dose. We calculate rates of severe SARS-CoV-2 disease between groups of individuals aged 60 and above, comparing those who received two doses at least 4 months previously to those who received the 3rd dose (stratified by the time from vaccination), and to those who received a 4th dose. The analysis shows that protection conferred by the 3rd dose against Omicron severe disease did not wane over a 7-month period. Moreover, a 4th dose further improved protection, with a severe disease rate approximately 3-fold lower than in the 3-dose cohorts.
Similar content being viewed by others
Introduction
Following a rise in cases due to the Delta variant and evidence of waning immunity against both confirmed infection and severe disease after 2 doses of the BNT162b2 vaccine1, Israel began administering a third BNT162b2 dose (booster) in July 2021. The 3rd dose was very effective against both confirmed infections and severe disease caused by the Delta variant2. However, recent studies have shown that the 3rd dose provides a much lower protection against infection with the Omicron variant (BA.1/BA.2) and that this protection wanes quickly3,4.
While a recently-administered 3rd dose was shown to be effective against severe disease caused by the Omicron variant5, it is not yet known how long this protection lasts. A study from the UK estimated 85% vaccine effectiveness against hospitalization for individuals 65 years of age or older 15 weeks after receiving the 3rd dose6. A CDC study showed waning of protection against hospitalization from 91% in the first 2 months after the 3rd dose to 78% by the fourth month7.
Here, we report on the protection against severe disease with the BA.1/BA.2 Omicron variants in individuals aged 60 or older up to 7 months after receiving a 3rd BNT162b2 dose, during the study period, January 16, 2022, to March 12, 2022. We used Poisson regression to estimate the rate of severe SARS-CoV-2 disease in the following cohorts: individuals who received the 2nd dose at least 4 months before the study ended, individuals who received the 3rd dose (divided into cohorts based on months from vaccination), and individuals who received the 4th dose. The regression model was adjusted for age group (60–69, 70–79, 80+), sex, sector (Arab, General Jewish, Ultra-Orthodox), and epidemiological week.
The follow-up was not extended since severe cases beyond the study period are affected not only by the vaccination but also by extremely widespread and largely undocumented prior infections due to increased use of antigen self-testing, which affected surveillance. In addition, the increased use of preventive medicines (e.g., Paxlovid and Molnupiravir) during this period raises further challenges in estimating vaccine effectiveness against severe disease.
The analysis shows that protection conferred by the 3rd dose against Omicron severe disease did not wane over a 7-month period. Moreover, a 4th dose further improved protection, with a severe disease rate approximately threefold lower than in the 3-dose cohorts.
Results
Table 1 reports basic descriptive statistics for the main cohorts of interest used in the analysis. The proportion of female days at risk is somewhat higher than that for males, with about 60% in the 2nd dose and 3rd dose up to 4 months cohorts and decreasing to about 55% for the 3rd dose over 5 months and the 4th dose cohorts. There are some differences in the age distribution across the different cohorts, with the proportion of days at risk among those aged 70–80 between 27 and 34% in the 2nd and 3rd dose cohorts and 42% in the 4th dose cohort. Similarly, the proportion of days at risk among those aged 80 and above is between 15 and 22% in the 2nd and 3rd dose cohorts and 26% in the 4th dose cohort. In addition, the distribution of sectors differs between the cohorts, with the proportion of days at risk among the General Jewish population being 63% in the 2nd dose cohort, between 77 and 85% in the 3rd dose cohorts, and 94% in the 4th dose cohort.
The rates of severe disease, and their ratios, estimated from the Poisson regression are summarized in Table 2 and Fig. 1. The protection conferred by the 3rd dose against Omicron severe disease did not show signs of waning over the first 7 months since vaccination, with rates of severe disease at ~4 per 100,000 risk days in all 3rd dose cohorts. This rate was approximately threefold lower than that in the 2nd dose cohort. The 4th dose further increased protection, with a severe disease rate approximately threefold lower than that in the 3rd dose cohorts.
Adjusted rates of severe illness per 100,000 risk days obtained from Poisson regression analysis for the study period January 16, 2022–March 12, 2022, adjusted for age category (60–69, 70–79, 80+), sex, sector, and exposure (based on epidemiological week). Error bars represent 95% confidence intervals, which are not adjusted for multiplicity.
Two sensitivity analyses, one allowing 21 (rather than the standard 14) days from a positive COVID test for severe disease to develop, and one stratified by age group, yielded rate estimates similar to those obtained in the main analysis.
Discussion
Studies have shown that the protection of a 2-dose BNT162b2 vaccine against both confirmed infection and severe disease wanes over time1. A recently-administered 3rd dose was shown to not just restore the initial level of protection of the 2-dose vaccine but exceed it8. Nevertheless, the level of protection of a 3rd BNT162b2 dose against confirmed Omicron BA.1/BA.2 infections was found to be substantially lower than against the Delta variant and to wane quickly. Our analysis shows that the effectiveness of the 3rd dose in preventing severe disease substantially exceeds that of the 2nd dose. Moreover, unlike the protection of the 2-dose vaccine, this protection does not appear to wane over the first 7 months since vaccination. Furthermore, our analysis regarding the protection against severe disease conferred by a 4th dose compared to the 3rd dose which showed an approximately threefold lower rate of severe disease in the 4th dose cohort aligns with a previous study9 that reported a 3.5-fold lower rate.
The results of the current study are aligned with studies that assess the immune levels and their durability following a 3rd BNT162b2 dose. Specifically, a large study from Israel following the serum of health careworks found that after the 3rd dose the humoral response declined slower than after the 2nd dose, started from a higher level in both IgG and neutralizing antibodies10. More so, the study also found that the avidity of the antibodies after the 3rd dose is higher than after the second dose10. In another study from Singapore showed that the total S-Ab/IgG/N-Ab levels increased post-booster compared to a 2nd dose, with IgG/N-Ab having a longer half-life11. Evidence from Japan also suggests that the 3rd dose conferred higher durable antibody titers12.
To date, this study reports the longest follow-up following administration of a 3rd dose, as Israel was the first country to administer a 3rd dose. Our analysis was based on a nationwide dataset that included data on more than 1 million elderly individuals. Severe cases were determined in a consistent way according to the NIH definition (see method) and were identified from daily reports issued by all hospitals in Israel. As such, the data were complete and representative of the entire elderly population in Israel.
The results of this study differ from the analysis of a recent CDC study7. The CDC study, which analyzed hospitalizations rather than severe cases, showed substantial waning 4 months after receiving the 3rd dose. However, there were only 765 individuals hospitalized over 4 months after receiving the 3rd dose. In addition, hospitalization is a measure that is not as specific as severe disease, hence can include a wide range of severity levels13.
This observational study has several limitations. First, there may be differences in risk of severe disease between the different cohorts. This limitation is addressed by adjusting for confounders such as age, sex, sector, and exposure. However, some risk differences could not be adjusted for. For instance, individuals who received the 3rd dose early may have been more likely to suffer from coexisting conditions. This could potentially bias the results toward under-estimation of vaccine effectiveness. Information on coexisting conditions is not recorded in the national database, and could not be adjusted for. Differences in coexisting conditions could also be associated with differential treatment with antiviral drugs such as Paxlovid and Molnupiravir, which could have affected the results14. In addition, individuals may have undocumented prior infections leading to hybrid immunity. As such hybrid immunity increases protection15, undocumented infections can severely bias the results. Moreover, the rate of prior infection is expected to be higher for people who received fewer vaccine doses as they are less protected, further biasing the results16.
Similar limitations arise in the comparison of the 3rd dose cohorts to the 4th dose cohort17. Specifically, individuals who tend to be more careful might also be more likely to take the 4th dose (leading to overestimation of the effect of the 4th dose). On the other hand, people who are at higher risk (e.g., due to comorbidities) might have a stronger tendency to both take the 4th dose and experience severe outcomes (leading to under-estimation of the effect of the 4th dose). Nevertheless, our results align with a previous study on the protection conferred by the 4th dose in Israel9 that overcomes the aforementioned behavioral biases. This latter study used an internal control group, comprised of individuals during days 3–7 after receiving the 4th dose, that is, before the booster dose was expected to have an effect, and obtained estimates for the protection against severe disease similar to ours.
During the study period, the dominant variant was Omicron sublineages BA.1/BA.2. Estimating the protection against Omicron BA.4/BA.5 severe disease and potential waning of the 3rd dose beyond 7 months is especially difficult due to missing infection data. Many, possibly most, individuals from all relevant cohorts (2nd, 3rd, and 4th dose) have likely been infected during the unprecedented BA.1/BA.2 Omicron wave. These infections are often undocumented as during this wave people increasingly self-tested at home using antigen tests (which have remained largely undocumented). In addition, during the BA.4/BA.5 wave, Israel greatly expanded the administration of Paxlovid and Molnupiravir to persons testing positive, reducing severe cases regardless of vaccination status. Thus, when other factors (e.g., ubiquitous hybrid immunity, preventive medication) reduce the risk of severe disease, the level of protection provided by vaccination and its waning become extremely difficult to estimate from population data. These challenges with respect to the estimation of protection during the BA.4/BA.5-dominant periods are shared globally. Hence, it is hard to obtain any reliable estimation of 3rd dose waning beyond the follow-up of 7 months reported in this paper.
The findings of the current study can support policy-making. The study suggests an extended duration of protection of the 3rd BNT162b2 dose against severe disease and the added protection of a 4th dose to risk groups. While it is still unclear what are the long-term effects of the 4th dose and the protection conferred by the new bivalent vaccines18, our findings can inform decisions regarding the timing of administration of additional booster doses depending on individual risk factors.
Methods
Description of the data
The Ministry of Health (MOH) in Israel collects all COVID-19-related variables in a central database. These include data on all institutional PCR and antigen tests (which are given free of charge) and results, vaccination dates and type of vaccine (almost all received the Pfizer-BioNTech vaccine), daily clinical status of all COVID-19-hospitalized patients, and COVID-19-related deaths. Specifically, the data used for conducting this study included vaccination dates, PCR and state-regulated rapid antigen tests (dates and results), hospital admission dates (if relevant), clinical severity status (severe disease or death), and demographic variables such as age, sex, and demographic group (General Jewish, Arab, ultra-Orthodox Jewish).
The data for the study were retrieved on March 26, 2022, with the outcome being severe illness due to Covid-19 during 14 days following a confirmed infection (defined using the NIH definition19 as a resting respiratory rate of more than 30 breaths per minute, oxygen saturation of <94% while breathing ambient air, or a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen of <300). Thus, the study period was set from January 16, 2022, to March 12, 2022, to allow for 14 days of follow-up from infection to severe disease. Those who died from COVID-19 during the first 14 days after confirmed infection were also counted as severe disease cases in our analysis. Surveillance of COVID-19-associated hospitalizations is continuously performed by the MOH. Data from all hospitals are updated daily, and often twice a day. In accordance with national guidelines, healthcare providers report all hospitalizations and deaths among individuals with laboratory-confirmed SARS-CoV-2 infection. Quality assurance of data was performed extensively over the course of the pandemic. The data are monitored daily by the MOH, and are continuously used for public health decision-making.
Study design and population
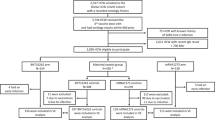
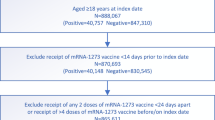
We conducted an observational study during Israel’s fifth wave, which was Omicron-dominant20, with both BA.1 and BA.2 sublineages present (initially BA.1 dominated, later BA.2 dominated). The study included persons who were 60 years of age or older, received their 2nd dose at least 4 months before the end of the study, did not have a documented infection by SARS-CoV-2 before the study period, had available data regarding sex and demographic sector, had not stayed abroad during the whole study period, and had not been vaccinated with a vaccine different from BNT162b2 before the study period (see Fig. 2).
The participants in the study included persons who were 60 years of age or older, who received 2nd dose at least 4 months before the end of the study, who were not infected by SARS-CoV-2 before the study period, had available data regarding sex and demographic sector, had not stayed abroad during the whole study period, and had not been vaccinated with a vaccine different from BNT162b2 before the study period.
Statistics and reproducibility
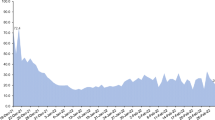
We estimated the adjusted rates of severe disease in nine cohorts: people who received only 2 doses of the vaccine and for whom at least 4 months had passed since receiving the second dose, people who received a 3rd vaccine dose, divided into 7 cohorts based on the number of months that passed since the administration of that dose, and people who recently received a 4th vaccine dose. Figure 3 shows the vaccination dynamics during the study period, and Table 2 reports basic descriptive statistics for the different cohorts. Individuals moved from one cohort to another after receiving a new vaccine or according to the time elapsed from the 3rd dose. Days at risk and severe disease cases occurring between vaccination (3rd or 4th dose) and 13 days after it were excluded, as they could not yet be associated with the new dose. Risk days for individuals who traveled abroad were not counted while they were outside of Israel.
A Poisson regression model was used to estimate the adjusted rate of severe disease per 100,000 risk days. The model included covariates adjusting for age group (60–69, 70–79, 80+), sex, sector (Arab, General Jewish, Ultra-Orthodox), and epidemiological week. We further conducted two sensitivity analyses. First, we included severe disease occurring up to 21 days following a confirmed infection. Second, we estimated the adjusted rates of severe disease within each age group (60–69, 70–79, and 80+).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the Israeli Ministry of Health but restrictions apply to the availability of these data and are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Israeli Ministry of Health. The numeric data for creating the figures in the paper are included in Supplementary Data 1.
Code availability
The code for creating the figures in the paper is included in Supplementary Code 1.
References
Goldberg, Y. et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 385, e85 (2021).
Bar-On, Y. M. et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 385, 1393–1400 (2021).
Abu-Raddad, L. J. et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 386, 1804–1816 (2022).
Patalon, T. et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat. Commun. 13, 3203 (2022).
Collie, S., Champion, J., Moultrie, H., Bekker, L.-G. & Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 386, 494–496 (2022).
Stowe, J., Andrews, N., Kirsebom, F., Ramsay, M. & Bernal, J. L. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study. Nat. Commun. 13, 5736 (2022).
Ferdinands, J. M. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 71, 255–263 (2022).
Amir, O. et al. Protection following BNT162b2 booster in adolescents substantially exceeds that of a fresh 2-dose vaccine. Nat. Commun. 13, 1971 (2022).
Bar-On, Y. M. et al. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 386, 1712–1720 (2022).
Gilboa, M. et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw. Open 5, e2231778 (2022).
Lau, C. S., Oh, M. L. H., Phua, S. K., Liang, Y.-L. & Aw, T. C. 210-Day Kinetics of Total, IgG, and Neutralizing Spike Antibodies across a Course of 3 Doses of BNT162b2 mRNA Vaccine. Vaccines 10, 1703 (2022).
Yamamoto, S. et al. Durability and determinants of anti-SARS-CoV-2 spike antibodies following the second and third doses of mRNA COVID-19 vaccine. 2022.11.07.22282054 Preprint at https://doi.org/10.1101/2022.11.07.22282054 (2022).
Sigal, A., Milo, R. & Jassat, W. Estimating disease severity of Omicron and Delta SARS-CoV-2 infections. Nat. Rev. Immunol. 22, 267–269 (2022).
Najjar-Debbiny, R. et al. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin. Infect. Dis. ciac443 (2022) https://doi.org/10.1093/cid/ciac443.
Goldberg, Y. et al. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 386, 2201–2212 (2022).
Kahn, R., Schrag, S. J., Verani, J. R. & Lipsitch, M. Identifying and Alleviating Bias Due to Differential Depletion of Susceptible People in Postmarketing Evaluations of COVID-19 Vaccines. Am. J. Epidemiol. 191, 800–811 (2022).
Canetti, M. et al. Six-Month Follow-up after a Fourth BNT162b2 Vaccine Dose. N. Engl. J. Med. 387, 2092–2094 (2022).
Chalkias, S. et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N. Engl. J. Med. 387, 1279–1291 (2022).
Clinical Spectrum. COVID-19 Treatment Guidelines https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/.
SARS-CoV-2 variants in analyzed sequences. Our World in Data https://ourworldindata.org/grapher/covid-variants-area.
Author information
Authors and Affiliations
Contributions
O.A., L.F., Y.G., A.H., M.M., and R.M. were responsible for the study design. All authors were responsible for writing and reviewing of the paper. O.A., O.B., Y.B., Y.G., and M.M. analyzed the data. N.A., S.A., O.B., Y.B., and Y.G. were responsible for collecting the data and for data management. N.A., O.A., S.A., O.B., L.F., A.H., and R.M. did the literature survey. O.A., Y.G., and M.M. developed the statistical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Institutional Review Board of the Sheba Medical Center.
Peer review
Peer review information
Communications Biology thanks Joe Grove, Douglas F. Lake and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Joao Valente.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amir, O., Goldberg, Y., Mandel, M. et al. Protection against Omicron BA.1/BA.2 severe disease 0–7 months after BNT162b2 booster. Commun Biol 6, 315 (2023). https://doi.org/10.1038/s42003-023-04669-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04669-6
- Springer Nature Limited