Abstract
Early exposures to anesthetics can cause long-lasting changes in excitatory/inhibitory synaptic transmission (E/I imbalance), an important mechanism for neurodevelopmental disorders. Since E/I imbalance is also involved with addiction, we further investigated possible changes in addiction-related behaviors after multiple ketamine anesthesia in late postnatal mice. Postnatal day (PND) 16 mice received multiple ketamine anesthesia (35 mg kg−1, 5 days), and behavioral changes were evaluated at PND28 and PND56. Although mice exposed to early anesthesia displayed normal behavioral sensitization, we found significant increases in conditioned place preference to both low-dose ketamine (20 mg kg−1) and nicotine (0.5 mg kg−1). By performing transcriptome analysis and whole-cell recordings in the hippocampus, a brain region involved with CPP, we also discovered enhanced neuronal excitability and E/I imbalance in CA1 pyramidal neurons. Interestingly, these changes were not found in female mice. Our results suggest that repeated ketamine anesthesia during neurodevelopment may influence drug reward behavior later in life.
Similar content being viewed by others
Introduction
As millions of children receive anesthesia for surgical or diagnostic procedures every year, anesthesia-induced neurotoxicity in the developing brain has received considerable research interest in recent decades1. Preclinical studies have shown that anesthetics commonly used in clinical settings may act as neurotoxins during neurodevelopment, inducing widespread neuronal cell death or excitatory/inhibitory (E/I) imbalance2,3,4,5. The disruption of E/I balance during the critical neurodevelopmental period is of considerable concern since E/I imbalance is an important mechanism for neurodevelopmental disorders6,7,8. In line with such concerns, studies that reported anesthesia-induced E/I imbalance also found long-term behavioral changes in activity, anxiety, sociability, learning, and memory5,9,10,11,12.
Based on preclinical studies, clinical studies have attempted to identify long-term negative effects of anesthesia in young children. While recent prospective clinical studies suggested that a short anesthetic exposure does not affect general intelligence13,14,15, the same studies also reported increased behavioral problems based on parental reports16. Other studies have reported possible associations between early anesthetic exposures and neurodevelopmental disorders (autism, ADHD)17,18. However, it is important to note that anesthesia-induced neurotoxicity was first identified in animal models without direct clinical evidence, making it difficult to speculate how this might manifest in children. Recent studies have acknowledged this fact and performed a wide range of tests to evaluate diverse cognitive and behavioral aspects. While these studies discovered changes in specific behaviors (motor and social linguistic performance, emotions) and executive functions16,19, many behavioral aspects remain unstudied. One possible, but less evaluated, the disorder is addiction. Previous studies have shown that changes in E/I synaptic transmission are deeply involved with addiction20,21. Single cocaine exposure has been shown capable of affecting AMPA/NMDA receptor currents22, and morphine exposure and withdrawal have been shown to affect GABA signaling23. Thus, it is possible that the synaptic changes due to early anesthesia exposures may also affect addiction behavior.
Ketamine, an intravenous anesthetic agent, is often used for sedation or anesthesia during short procedures in pediatric patients due to its’ lack of respiratory depression and excellent analgesic effects24. However, unlike other anesthetic agents, ketamine is also classified as a ‘dissociative anesthetic’. By disconnecting the thalamo-neocortical and limbic systems, ketamine can induce hallucinations and altered sensory states. As a result of these attributes, ketamine has come to be used as a recreational drug at sub-anesthetic doses, leading to drug abuse25. Since ketamine exposures during neurodevelopment induce E/I imbalance26,27, and ketamine itself has addictive properties, we hypothesized that repeated ketamine anesthesia during neurodevelopment might affect addiction behavior later in life.
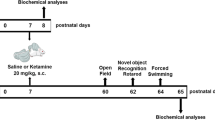
To test our hypothesis, late postnatal mice (postnatal day 16 [PND16]) received multiple anesthetic treatments with ketamine (35 mg kg−1, i.p.) for 5 consecutive days. Long-term changes in addiction-related behaviors were evaluated by measuring behavioral sensitization and conditioned place preference (CPP) to low-dose ketamine (20 mg kg−1, i.p.) and nicotine (0.5 mg kg−1 s.c.) in adolescent (PND28) and adult (8 weeks old) mice. Although there was no change in behavioral sensitization, we discovered increases in place preference for both ketamine and nicotine in male mice who had received early ketamine anesthesia. Interestingly, such behavioral changes did not occur in female mice. To further identify possible mechanisms underlying the changes in drug reward but not sensitization, we performed transcriptome analysis and whole-cell recordings in the hippocampus, a brain region deeply involved with CPP28,29,30. Our results suggest that exposures to ketamine during neurodevelopment can cause long-lasting changes in drug reward, and that sex must be regarded as an important biological variable when studying the early effects of anesthesia.
Results
Development of acute tolerance after repeated ketamine anesthesia in late postnatal male mice
As previous studies suggest that multiple anesthetic exposures may affect neurodevelopment15,16,19, we injected late postnatal mice with an anesthetic dose of ketamine (35 mg kg−1, i.p.) for 5 consecutive days (PND16–20). The anesthetic dose of ketamine was based on our previous study, where we found that higher doses occasionally caused prolonged anesthesia or mortality (>40 mg kg−1, i.p)26. Unlike other anesthetic agents, clinical studies have reported acute tolerance after repeated ketamine anesthesia in children31,32. Thus, by measuring the loss-of-righting (LOR) reflex, we first examined whether acute tolerance also developed after repeated ketamine anesthesia in young mice. Although ketamine induced a short duration of anesthesia on day 1 (230 ± 203.4 seconds), the duration of anesthesia significantly declined with following injections (Fig. 1a). In fact, due to the development of acute tolerance, LOR did not develop in most mice after day 3 (Fig. 1a). While our results clearly show rapid development of ketamine tolerance in late postnatal mice, there was no effect on general growth, as ketamine-injected mice showed similar weight gain compared with saline-injected mice (Fig. 1b).
a PND16 male mice were injected with an anesthetic dose of ketamine for 5 consecutive days. The duration of ketamine anesthesia, measured as the LOR reflex duration, was significantly reduced by repeated injections (acute tolerance) (p = 0.001, repeated measures [RM]-ANOVA; n = 12). b Normal weight gain in mice that received repetitive ketamine anesthesia (p = 0.115, RM-ANOVA; Control, n = 15; Anesthesia, n = 12). c–g Early repeated ketamine anesthesia does not affect the development of behavioral sensitization to low-dose ketamine. c, d Schematic diagram of the experimental design. A behavioral sensitization test was performed 1 week after anesthesia injections. e Early ketamine anesthesia did not affect baseline activity at PND28 (p = 0.158, Student’s t test; Control-Saline, n = 12; Anesthesia Saline, n = 11). f Behavioral sensitization to repeated low-dose ketamine developed in both groups (one-way ANOVA with Tukey multiple comparisons; Control-Ketamine, n = 12; anesthesia ketamine, n = 12). g The development of behavioral sensitization was comparable between the two groups (p = 0.583, RM-ANOVA; Control-Ketamine, n = 12; Anesthesia ketamine, n = 12). Values are presented as means ± SD (n.s., not significant; ***p < 0.001).
Behavioral sensitization to low-dose ketamine was not affected by early repeated ketamine anesthesia in male mice
To investigate whether the acutely developed tolerance can influence addiction-related behaviors later in life, we first examined behavioral sensitization to low-dose ketamine 1 week after anesthesia treatment (Fig. 1c, d). Previous studies have shown that repeated injections of low-dose ketamine (20 mg kg−1, i.p.) induce behavioral sensitization33,34, a progressively enhanced behavioral response following repetitive administration of abusive drugs. Behavioral sensitization has been suggested to reflect neuronal adaptations following substance abuse35. After confirming that the baseline activity was not affected by multiple ketamine anesthesia (Control-Saline group vs. Anesthesia-Saline group, Fig. 1e), mice were repeatedly injected with ketamine in a 4-day interval (Fig. 1d). Behavioral sensitization occurred in both groups, as total activity significantly increased after five identical ketamine injections (Fig. 1f). We also found that the level of sensitization was comparable between the two groups (Fig. 1f, g). Thus, although repeated ketamine anesthesia-induced rapid tolerance in late postnatal mice, such acute changes did not affect behavioral sensitization to low-dose ketamine.
Place preference for low-dose ketamine and nicotine is enhanced 1 week after repeated ketamine anesthesia in male mice
Another commonly used behavioral test for assessing the motivational effects of addictive drugs is the conditioned place preference (CPP) test, which evaluates contextual associations in reward-related behavior36. Thus, we next investigated whether early anesthesia influenced place preference for low-dose ketamine (20 mg kg−1, i.p.) (Fig. 2a). Place preference developed regardless of anesthesia history, as evidenced by significant increases in the duration spent in the white (ketamine-paired) chamber after conditioning (Fig. 2b). However, mice in the Anesthesia group developed more robust place preference compared with mice in the Control group (Fig. 2c, Supplementary Fig. 1).
a–c Early repeated ketamine anesthesia increased low-dose ketamine-induced place preference 1 week after anesthesia injections (ketamine CPP). a Experimental scheme of ketamine CPP. b Time spent in the ketamine-paired (white) chamber was significantly increased after conditioning in both groups (Control, p = 0.007; paired t test; n = 26 mice; Anesthesia, p < 0.001; paired t test; n = 27 mice). c Summary graph comparing ketamine CPP scores between groups (p = 0.0496, Welch ANOVA; control, n = 26 mice; Anesthesia, n = 27 mice). d, e Early repeated ketamine anesthesia increased nicotine-induced place preference 1 week after anesthesia injections (nicotine-CPP). d Time spent in the ketamine-paired (white) chamber was significantly increased after conditioning in the Anesthesia group (p < 0.001) but not in the Control group (p = 0.056) (paired t test; control, n = 16 mice; Anesthesia, n = 16 mice). e Summary graph comparing nicotine-CPP scores between groups (p = 0.0192, Welch ANOVA; control, n = 16 mice; Anesthesia, n = 16 mice.). Values are presented as means ± SD (n.s., not significant; *p < 0.05, **p < 0.05, ***p < 0.001).
To further investigate whether early ketamine anesthesia can also affect the motivational effects of different drugs of abuse, we next repeated the CPP test using nicotine (0.5 mg kg−1, s.c.) (Fig. 2d, e). Interestingly, nicotine-induced place preference occurred only in mice in the Anesthesia group (Fig. 2d). There was also a significant difference in CPP scores between the two groups (Fig. 2e). These results suggest that early repeated ketamine anesthesia selectively enhances place preference to abusive drugs without affecting behavioral sensitization.
Place preference for nicotine continues to be enhanced after a prolonged drug-free period in male mice
We next investigated whether increases in place preference persist after a more prolonged drug-free period by performing the CPP test 5 weeks after anesthesia treatment (8 weeks old) (Fig. 3a). Unexpectedly, the same CPP protocol with low-dose ketamine did not induce place preference in either group at this age (Fig. 3b, c). As this may be attributable to the lower susceptibility to substance abuse at older ages37, we repeated the CPP test with a longer conditioning period (14 days). However, place preference still did not develop (Supplementary Fig. 2). Another possibility is that ketamine-induced CPP is not as robust as other addictive drugs, since a previous study has also reported negative results38. The simplicity of our CPP apparatus may also be involved. While most CPP tests use various visual and tactile cues to distinguish chambers, we used a more simplified version (black and white chambers). Although such simplicity may suppress the development of place preference, we considered it could increase the sensitivity for discovering differences in place preference induction. Such simplicity may also contribute to the lack of place preference for nicotine in the Control group (Figs. 2d, 3d). Importantly, place preference for nicotine still developed in mice that received early ketamine anesthesia (Anesthesia group) (Fig. 3d). Although the CPP score for nicotine was comparable between control and anesthesia mice (Fig. 3e), our results suggest that the effects of early repeated ketamine anesthesia may persist into adulthood.
a–c Early repeated ketamine anesthesia does not affect place preference for low-dose ketamine in adult mice (ketamine CPP). a Experimental scheme of ketamine CPP performed in adult male mice. b Place preference to low-dose ketamine does not occur in both Control (p = 0.074) and anesthesia (p = 0.183) groups (paired t test; control, n = 26; anesthesia, n = 25). c Summary graph comparing ketamine CPP scores between groups (p = 0.858, student’s t test; control, n = 26; anesthesia, n = 25). d–e Nicotine-induced place preference occurred only in mice that received early repeated ketamine anesthesia (nicotine-CPP). d Time spent in the nicotine-paired (white) chamber was significantly increased after conditioning in the anesthesia (p = 0.011) but not in the control (p = 0.172) group (paired t test; control, n = 18, anesthesia, n = 17). e Summary graph comparing nicotine-CPP scores between groups (p = 0.894, Welch ANOVA; control, n = 18, Anesthesia, n = 17). Values are presented as means ± SD (n.s., not significant; *p < 0.05).
Repeated ketamine anesthesia increases the expression of genes encoding axon-localized proteins that regulate neuronal excitability in the hippocampus of male mice
Given that the mesolimbic dopaminergic system is deeply involved in addiction, we investigated whether early ketamine anesthesia increased the level of dopamine and its metabolite, 3,4-dihydroxyphenylacticacid (DOPAC), in the mesolimbic circuit using high-performance liquid chromatography (HPLC). These analyses showed that early repeated ketamine did not increase the levels of dopamine or DOPAC, measured directly after the CPP test (Supplementary Fig. 3). In fact, the level of dopamine was decreased in the ventral tegmental area (VTA). However, it is important to note that early ketamine anesthesia affected CPP, but not behavioral sensitization. The CPP test measures reward-related learning and memory of drug-context associations36,39, a process with deeply involves the hippocampus28,29,30. Thus, we performed a transcriptome analysis (RNA-sequencing) of the hippocampus to identify possible mechanisms underlying the increase in place preference. A total of 38 differentially expressed genes (DEGs; 34 upregulated and 4 downregulated) were identified in mice that received repeated ketamine anesthesia compared with mice in the control group (Fig. 4a, Supplementary Tables 1–3, Supplementary Data 1). However, the majority of changes were less than twofold, suggesting that early ketamine anesthesia does not induce major changes in expression at the single-gene level. To evaluate whether these DEGs are involved in similar biological pathways, we next performed functional enrichment analysis using Enrichr (GO/KEGG pathway analysis). Despite the relatively low number of DEGs, we discovered significant associations with the cellular component categories, “axon initial segment”, “main axon”, and “node of Ranvier” (Fig. 4b, Supplementary Tables 4–6). There were no changes in molecular function or biological process categories (Fig. 4b, Supplementary Table 5). To further identify changes in biological functions that are driven by large numbers of genes with moderate, but coordinated, changes in expression, we also performed a gene set enrichment analysis (GSEA) using the entire list of genes. Similar to the results of the Enrichr functional enrichment analysis, we found upregulation of genes involved in the regulation of neuronal activity whose encoded products are localized to neuronal axons (Supplementary Tables 7–10). GSEA results also suggested that upregulation occurred mostly in pyramidal neurons of the hippocampus (Supplementary Table 11).
a, b RNA-sequencing was performed with hippocampal samples 1 week after repeated ketamine anesthesia or saline injections in male mice. a Volcano plot of DEGs, shown as blue (decreased expression) and red (increased expression) circles. b Gene ontology (GO) analysis of DEGs. Significant terms are highlighted in red (Axon initial segment, p < 0.001; Main axon, p = 0.007; Node of Ranvier, p = 0.034; n = 4 for each group). c–f Whole-cell recordings were performed 1 week after repeated ketamine anesthesia in hippocampal CA1 pyramidal neurons of male mice. c Enhanced intrinsic excitability after early repeated ketamine anesthesia. The number of action potentials was measured after injecting a series of depolarizing currents under current-clamp conditions (current injection = 60 pA, p = 0.034; 70 pA, p = 0.018; 80 pA, p = 0.006; 90 pA, p = 0.005; 100 pA, p = 0.009; 110 pA, p = 0.029; Student’s t test; control, n = 6 mice [total 20 cells]; anesthesia, n = 7 mice [total 20 cells]. d, e Altered excitatory and inhibitory spontaneous synaptic transmissions in hippocampal CA1 pyramidal neurons. d Anesthesia group showed increased frequency (Welch ANOVA, p = 0.039), but normal amplitude (Kruskal–Wallis test, p = 0.908) of sEPSCs (control, n = 7 mice [total 21 cells]; anesthesia, n = 8 mice [total 21 cells]). e Anesthesia group showed decreased frequency (Student’s t test, p = 0.010) but normal amplitude (Student’s t test, p = 0.418) of sIPSCs in hippocampal CA1 pyramidal neurons (control, n = 6 mice [total 22 cells]; anesthesia, n = 6 mice [total 22 cells]). f Spontaneous action potentials (sAP) are significantly increased after early repeated ketamine anesthesia. sAPs were measured under I = 0 mode (p = 0.010, Kruskal–Wallis test; Control, n = 6 mice [total 21 cells]; anesthesia, n = 6 mice [total 24 cells]). Values are presented as means ± SD (n.s., not significant; *p < 0.05; **p < 0.01).
Repeated ketamine anesthesia affects intrinsic excitability, E/I balance, and neuronal activity in CA1 hippocampal neurons of male mice
Based on our transcriptome analysis (RNA-sequencing) results, we next evaluated whether repeated ketamine anesthesia affects the electrophysiological properties of CA1 hippocampal pyramidal neurons. Changes in intrinsic neuronal excitability were first evaluated by injecting a series of depolarizing currents under current-clamp conditions40. Although there were no differences in resting membrane potential, input resistance, or action potential firing thresholds between groups (Supplementary Fig. 4), we discovered a significant increase in the number of action potential firings (current-firing curve) in the Anesthesia group (Fig. 4c). We also found significant changes in spontaneous synaptic transmission. Repeated ketamine anesthesia increased the frequency of sEPSCs without affecting their amplitude (Fig. 4d), while decreasing the frequency of sIPSCs (Fig. 4e). However, there were no differences in the amplitude of both sEPSCs and sIPSCs (Fig. 4d, e). Interestingly, such changes in synaptic transmission were activity-dependent, as the addition of tetrodotoxin (TTX), a voltage-gated sodium channel blocker, eliminated the changes (mEPSCs/mIPSCs) (Supplementary Fig. 5a–d). Since our results suggest an increase in neuronal activity, we next evaluated changes in spontaneous neuronal firing under current-clamp (I = 0 mode). Although rarely discovered in the control group, spontaneous action potentials were significantly increased in mice that received repeated ketamine anesthesia (Fig. 4f). Our RNA-seq results and electrophysiology data suggest that early repeated ketamine anesthesia induces long-lasting changes in hippocampal activity, which may act as an important mechanism for the increases in place preference behavior.
Early repeated ketamine anesthesia does not enhance ketamine-induced CPP and hippocampal excitability in female mice
Sex is now recognized as an important biological variable in neuroscience, and sex-dependent changes in cocaine addiction behavior were recently reported after prolonged antidepressant ketamine treatment in young mice41. Thus, we further investigated the effects of early repeated ketamine anesthesia in late postnatal female mice. Similar to male mice, acute tolerance developed during repeated ketamine anesthesia (Supplementary Fig. 6a), and there was no difference in behavioral sensitization to low-dose ketamine 1 week after anesthesia (Supplementary Fig. 6b). However, we found differences in the CPP test results between male and female mice. Although place preference for low-dose ketamine occurred in both groups 1 week after injections (Fig. 5a), in contrast to male mice, place preference for low-dose ketamine was decreased in the Anesthesia group (Fig. 5b). Also, female mice did not develop place preference to nicotine, regardless of anesthesia history (Supplementary Fig. 7). While our results suggest that early ketamine anesthesia does not enhance drug reward behavior in female mice, it is important to recognize that CPP measures drug reward by evaluating the association of a specific context and drug stimulus. Thus, to imply that female mice are resistant to the increase in drug reward induced by early ketamine anesthesia, it is necessary to confirm that non-drug-associated memory is also not affected. By performing the Barnes maze and fear chamber test, we further confirmed that non-drug-associated learning and memory were not affected in female mice (Supplementary Fig. 8).
a, b Early repeated ketamine anesthesia reduces low-dose ketamine-induced place preference 1 week after anesthesia injections in female mice (ketamine CPP). a Time spent in the ketamine-paired (white) chamber was significantly increased after conditioning in both groups (control, p < 0.001; paired t test; n = 25; Anesthesia, p = 0.010; paired t test; n = 27). b Summary graph comparing ketamine CPP scores between groups (p = 0.047, Student’s t test). c–f Whole-cell recordings were performed in hippocampal CA1 pyramidal neurons from female mice 1 week after repeated ketamine anesthesia. c Normal intrinsic excitability after early repeated ketamine anesthesia in female mice. The number of action potentials was measured after injecting a series of depolarizing currents under current-clamp conditions (Kruskal–Wallis test and Student’s t test; control, n = 6 mice (total 20 cells); anesthesia n = 6 mice (total 21 cells)). d, e Repeated ketamine anesthesia does not affect spontaneous excitatory synaptic transmission (Welch ANOVA, amplitude: p = 0.190; Student’s t test, frequency: p = 0.698; control, n = 7 mice (total 21 cells); anesthesia, n = 8 mice (total 21 cells)) and spontaneous inhibitory synaptic transmission in female mice (Student’s t test, amplitude: p = 0.833; Student’s t test, frequency: p = 0.643; Control, n = 6 mice (total 22 cells); anesthesia n = 7 mice [total 21 cells]). f Early repeated ketamine anesthesia does not affect spontaneous action potentials (sAPs). sAPs were measured under I = 0 mode (p = 0.600, Kruskal–Wallis test; control, n = 6 mice [total 22 cells]; anesthesia, n = 7 mice [total 20 cells]). Values are presented as means ± SD (*p < 0.05; ***p < 0.001; n.s., not significant).
In line with the sex differences in drug reward behavior, we also found that repeated ketamine anesthesia during neurodevelopment did not affect the electrophysiologic properties of hippocampal neurons in female mice (Fig. 5c–f). We found no changes in intrinsic excitability (Fig. 5c, Supplementary Fig. 9), synaptic transmission (Fig. 5d, e, Supplementary Fig. 10), and spontaneous neuronal activity (Fig. 5f). Although we did not directly investigate the causality between electrophysiological changes and place preference behavior, such sex-differences support our hypothesis that the changes in the hippocampus are involved in the increase in place preference after early ketamine anesthesia.
Discussion
Recent clinical studies have suggested that exposures to anesthetics during neurodevelopment may affect specific behaviors rather than affecting global cognitive function. In the present study, we investigated possible changes in addiction-related behaviors after repeated ketamine anesthesia in late postnatal mice. We found that repeated ketamine anesthesia did not affect behavioral sensitization, but increased place preference for low-dose ketamine and nicotine. Interestingly, such changes in drug-associated reward behavior only occurred in male mice. In fact, place preference for low-dose ketamine was reduced in female mice that received early ketamine exposures. Consistent with the increase in place preference in male mice, changes in intrinsic excitability, spontaneous synaptic transmission, and a number of spontaneous action potentials in hippocampal pyramidal neurons also occurred only in male mice.
While few studies have previously investigated the effects of early ketamine exposures on reward behavior41,42,43, the present study differs in several important aspects. The first important difference is the dose and duration of ketamine treatment. Previous studies evaluated the consequences of antidepressant doses of ketamine (10–20 mg kg−1) which was injected for a prolonged period (10–15 days), whereas we used a higher anesthetic dose of ketamine (35 mg kg−1) for a much shorter period (5 days). Secondly, we evaluated changes in both behavioral sensitization and place preference, unlike previous studies which only measured a single behavior. The third and most important difference is that we identified a plausible mechanism for the male-dependent increase in place preference by discovering male-dependent changes in hippocampal neuronal activity. Recent studies suggested that the dorsal hippocampus plays an essential role in the acquisition of contextual memory associated with abusive drugs such as nicotine and cocaine28,29,30. Our results are in line with such previous studies, as spontaneous neuronal activity was increased in the hippocampus after repeated ketamine anesthesia in male mice.
Although sex is recognized as an important biological factor44, we did not initially perform experiments in female mice. This was based on our recent study, where we reported non-sex-dependent changes after repeated ketamine anesthesia (two-way ANOVA)26. However, another recent study reported conflicting results by showing that repeated ketamine treatment in young mice enhanced place preference for cocaine only in male mice41. Interestingly, we also discovered increased drug reward behavior and enhanced hippocampal activity only in male mice. Our results, however, are in contrast with other preclinical studies reporting enhanced sensitivity to ketamine in female rodents45,46,47,48. For instance, studies have reported that the dose-response threshold for ketamine’s antidepressant effect was lower in female mice45,46,47. Another study reported that behavioral sensitization developed only in female mice when using ketamine 2.5 mg kg−1 48. However, studies using a relatively higher dose of ketamine (10 mg kg−1) have reported longer antidepressant effects in male mice46, and that prolonged injections induced antidepressant effects only in male mice49. Based on these results, it is possible that the sex differences in ketamine sensitivity are dose-dependent. Unfortunately, further studies are necessary as only a few studies have focused on higher doses of ketamine (20 mg kg−1)38.
Another interesting finding was the increased expression of genes encoding axon-localized proteins involved with neuronal activity after early ketamine exposures. Early studies have reported increased dendritic spinogenesis after exposure to anesthetics, including ketamine, in late postnatal mice27. However, our transcriptome analysis of the hippocampus after ketamine anesthesia revealed expression changes only in genes encoding axon-localized proteins. One possible explanation for such differences is the timing of the experiments. Because the increase in dendritic spines after ketamine exposure is transient27, such changes may not be detected by RNA-sequencing 1 week after anesthetic exposure. The number of ketamine anesthetic exposures may also be involved, given that previous studies evaluated the effects of a single exposure27. While additional studies at different time points using diverse anesthesia protocols are necessary, our results suggest that future studies may need to focus on long-lasting axonal changes rather than transient increases in dendritic spines.
The developmental stage of the animal is an important factor for anesthesia-induced neurotoxicity2,3,4,12. Although the majority of preclinical studies have been performed at PND7, it has been suggested that this age is comparable to a third-trimester human fetus from a neurodevelopment standpoint (www.translatingtime.org)50,51. This may cause discrepancies between preclinical and clinical studies since the majority of clinical studies focused on infants or very young children. Our study performed ketamine anesthesia at PND16–20, an age that has been suggested to be comparable to a 6 to 9 months-old human infant50,51. However, because it is difficult to directly compare neurodevelopment between humans and rodents, additional studies at diverse ages are necessary to understand the potential effects of early anesthesia.
There are several important limitations in the present study. The first limitation is the lack of evidence confirming a direct causative relationship between the increased hippocampal excitability and changes in reward behavior. Although this can be achieved using direct drug treatment through surgically implanted cannulas52 or optogenetic/chemogenetic inhibition53,54, we were unable to perform such studies due to a lack of expertise and equipment. Secondly, we cannot exclude the role of other brain regions. Although we focused on the hippocampus since early ketamine exposures only affected place preference (not behavioral sensitization), hippocampal activity is also involved with dopamine release from VTA dopaminergic neurons55. Thus, additional studies in diverse brain regions involved with an addictive behavior may provide additional evidence regarding the changes from early ketamine exposures. Thirdly, the interval between early ketamine anesthesia and the CPP tests may not have been sufficient to evaluate long-term changes in drug reward. CPP tests were performed at PND28 and PND56. However, these ages may be comparable to a 16-month infant and a 2-year-old child from a neurodevelopmental standpoint50,51. Thus, although considered adolescent and adult mice, these ages may not be sufficient to evaluate the long-term effects of early ketamine anesthesia. Fourthly, the use of a fixed ketamine dose (35 mg kg−1) for repetitive anesthesia despite the development of acute tolerance may also act as a limitation. As the purpose of the study was to evaluate the long-term consequences of early ketamine anesthesia, it may have been reasonable to increase the dose of ketamine when LOR did not occur. However, this could complicate data analysis, since each mouse would have received different doses of ketamine. Lastly, although we discovered changes in place preference after repeated ketamine anesthesia, the CPP test is not a direct animal model for addiction behavior. The CPP test is an animal model of drug reward, and the drug is passively administered39. In contrast, during the self-administration test, the subject must learn a task that produces acute effects from self-drug administration. Thus, the self-administration paradigm is considered a more direct measurement of drug reinforcement and addiction39. Future studies using the self-administration paradigm are necessary to address the effects of early repeated ketamine anesthesia on addictive behavior.
In conclusion, by evaluating late postnatal mice exposed to repeated ketamine anesthesia, we provide evidence that early anesthetic exposures using ketamine increased hippocampal activity, resulting in long-term change in reward-related learning and memory only in male mice. Our results raise the possibility that repeated use of ketamine for anesthetic purposes may affect abusive drug use later in life. Future studies must recognize that early anesthetic exposures may affect diverse behaviors, and that sex should always be considered an important biological variable.
Methods
Animals
All procedures were approved by the Committees on Animal Research at Chungnam National University Hospital (Daejeon, South Korea, CNUH-018-P0006, 202009A-CNU-155). C57BL/6 mice (RRID: MGI:5659186, Damul Science, Daejeon, Korea) were housed at a relative temperature of 22 °C and humidity of 40%, in a 12-hour light/dark cycle, and allowed free access to food and water. Mice were weaned at postnatal day (PND) 21 and housed in groups of three to five per cage.
Anesthesia
Ketamine hydrochloride (Huons, South Korea, 50 mg ml−1) was diluted to 2.5 mg ml−1 with normal saline before injections. Mice were randomly divided into two groups at PND16 (Control and Anesthesia group). Mice in the Anesthesia group received daily intraperitoneal injections of ketamine (35 mg kg−1) for 5 consecutive days26. Although the majority of preclinical studies exposed young animals to 3 episodes of anesthesia, ketamine is often used outside the operating room for more frequently repeated short-term procedures or radiographic examinations31,32. Thus, we increased the number of daily injections to 5 days. Mice were placed in an empty cage containing fresh bedding (36 °C) for 30 minutes after injections. Mice in the Control group received identical treatment with normal saline.
Anesthesia duration, defined as the time from intraperitoneal injection to the regaining of the righting reflex, was measured using a subset of mice. Once the mice lost the ability to right themselves, mice were placed in a supine position on a flat surface. Righting reflex was defined as the ability to right themselves on all four paws three times within 30 seconds56.
Behavior tests
Behavioral tests (behavioral sensitization, conditioned place preference test, Barnes maze, fear chamber test) were performed in a sound-attenuated room and video-recorded by a blind experimenter. Video recordings were later automatically analyzed using tracking software (Ethovision XT; Noldus Information Technology, Netherlands). Mice with growth disturbance due to malocclusion or severe injury due to fighting in group-housed mice were excluded.
Behavioral sensitization
Behavioral sensitization (enhanced locomotor activity after repeated exposures to drugs of abuse) was measured using the open field box (width/height/length, 40 cm). Mice were placed in the center of the open field box after i.p. injection of low-dose ketamine (20 mg kg−1) or an equal volume of saline. The general activity was measured for 60 minutes. The experiment was repeated 5 times, in a 4-day interval57. Behavioral sensitization was evaluated by measuring the increase in locomotor activity.
Conditioned place preference (CPP) test
The CPP apparatus consisted of three chambers connected with small openings (7 × 7 cm). The two main compartments (width/height/length, 15/20/20 cm) differed in color (black vs white) and were connected by a small gray chamber (width/height/length, 10/20/10 cm). The CPP test was performed using a standard CPP protocol including a pre-conditioning phase, a conditioning phase, and a post-conditioning phase. During the preconditioning phase (day 1–2), mice were placed in the gray chamber and were allowed to explore the full region of the CPP apparatus freely for 15 minutes. The sessions were video-recorded and the time spent in the white chamber was measured on day 2 (pre-conditioning duration). The conditioning phase was conducted for 8 consecutive days, with one conditioning trial per day. For ketamine CPP, mice received low-dose ketamine (20 mg kg−1) and were confined in the white chamber for 30 minutes (days 3, 5, 7, 9). Between low-dose ketamine injections, mice were injected with an equal volume of saline and confined in the black chamber for 30 minutes (days 4, 6, 8, 10). The post-conditioning phase was performed the following day (day 11). Mice were allowed to freely explore the full region of the CPP apparatus freely for 15 minutes, and the time spent in the white (ketamine-paired) chamber was measured (post-conditioning duration). CPP score was calculated using the time spent in the white (ketamine-paired) chamber during pre/post-conditioning phases (CPP score = ‘post-conditioning duration’—‘pre-conditioning duration’). The same protocol was used for the nicotine-CPP test. Nicotine (nicotine hydrogen tartrate salt, Sigma-Aldrich, St Louis, USA) was subcutaneously injected (0.5 mg kg−1).
Barnes maze test
Spatial learning and memory were evaluated by performing the Barnes maze test58. The maze consisted of a circular platform (92 cm diameter) with 20 holes (5 cm diameter) containing a hidden escape box (Scitech Korea Inc., Korea). The platform was elevated 95 cm above the floor and was surrounded by three spatial cues. The test was performed in a bright room and a buzzer (80 dB) was used to motivate mice to the enter escape box.
Mice were first placed in the escape box for 3 minutes (habituation). Following habituation, mice were placed in the middle of the maze using a black-colored ‘start chamber’. 10 seconds after the buzzer was turned on, the start chamber was removed and mice were trained to enter the escape box. The buzzer was turned off once the mice entered the escape box, and the mice remained in the box for 2 minutes (pre-training trial). 1 hour after pre-training, mice received the first training session. Training sessions (three sessions daily) were performed for 4 days. During the trial sessions, the buzzer was turned off once the mice entered the escape box and the mice remained in the escape box for 1 minute. Mice were gently guided if they did not enter the escape box within 3 minutes. To avoid the possibility of intra-maze cues, the maze was rotated each day. After the training sessions, the escape box was removed and mice were placed in the maze for 45 seconds (probe trial).
Learning and memory were compared by measuring the latency, path length, and a number of errors for mice to enter the escape box. Total latency was defined as the duration until mice entered the escape box; total path length was the total moved distance before entering the escape box; total errors were the number of noses pokes over holes irrelevant to the escape box before mice entered the escape hole. As mice may not enter the escape box despite finding the correct escape hole, we also measured the latency, path length, and a number of errors until the mice first encountered the escape hole (primary latency, primary path length, primary errors). Latency, errors, and path length were manually measured by an experimenter blinded to the condition of the mice, while distance was measured using tracking software (Ethovision XT).
Fear chamber test
Contextual-fear memory was measured using the fear chamber test (Coulbourn Instruments, MA, USA)12. Fear conditioning was performed by applying three trials of a stimulus (1 mA electric shock, 1 minute interval), 5 minutes after placing the mice in the fear chamber. Contextual-fear memory was analyzed the following day after placing mice in the same chamber for 5 minutes. The freezing duration was automatically measured using the FreezeFrame software (Coulbourn Instruments).
High-performance liquid chromatography (HPLC)
The level of dopamine and 3,4-dihydroxyphenylacticacid (DOPAC) in several brain regions were measured by high-performance liquid chromatography (HPLC, 1260 Infinity system, Agilent Technologies, CA, USA) with electrochemical detection. After brief anesthesia with sevoflurane, the nucleus accumbens (NAcc), ventral tegmental area (VTA), and striatum were dissected in ice-cold PBS and stored at −80 °C prior to analysis. Brain tissues were homogenized in 0.1 M perchloric acid and centrifuged at 14,000 rpm for 15 minutes. The supernatants were injected using an autosampler and eluted through a SunFire C18 column (4.6 × 100 mm × 5 μm; Waters Corporation, MA, USA). Peaks were detected using an ESA Coulochem III electrochemical detector and analyzed using ChemStation software (Agilent Technologies, CA, USA). The concentrations of dopamine and DOPAC were normalized to total protein per sample determined by a protein assay kit (Thermo Scientific, MA, USA).
RNA-sequencing
RNA was extracted from the hippocampus of mice 1 week after repeated saline injections or ketamine anesthesia (4 mice for each group). Total RNA was obtained using the RNeasy Lipid Tissue Mini Kit (QIAGEN, Germany, Cat. 74804) and stored at −80 °C. Library preparation, cluster generation, and sequencing were performed by Macrogen Inc. (Seoul, Korea): Library kit, TruSeq Stranded Total RNA LT Sample Prep Kit (Gold); Library protocol, TruSeq Stranded Total RNA Sample Prep Guide, Part #15031048 Rev. E; Reagent, NovaSeq 6000 S4 Reagent Kit; Sequencing protocol, NovaSeq 6000 System User Guide Document #1000000019358 v02, Sequencer type, NovaSeq 6000; Sequencing Control Software, 1000000019358 v02.
RNA-Seq analysis
Transcript abundance was estimated with Salmon (v1.1.0)59 in Quasi-mapping-based mode onto the Mus musculus genome (GRCm38) with GC bias correction (--gcBias). Quantified gene-level abundance data was imported to R (v.3.5.3) with the tximport60 package and differential gene expression analysis was carried out using R/Bioconductor DEseq2 (v1.30.1)61. Normalized read counts were computed by dividing the raw read counts by size factors and fitted to a negative binomial distribution. The P values were adjusted for multiple testing with the Benjamini–Hochberg correction. Genes with an adjusted P value of less than 0.05 were considered as differentially expressed. The Gene Ontology (GO) enrichment analyses were performed using Enrichr software62,63. Mouse gene names were converted to human homologs using the Mouse Genome Informatics (MGI) database (http://www.informatics.jax.org/homology.shtml). Gene Set Enrichment Analysis (GSEA) (http://software.broadinstitute.org/gsea)64 was used to determine whether a priori-defined gene set would show statistically significant differences in expression between control- and ketamine anesthesia treated mice. Enrichment analysis was performed using GSEAPreranked (gsea-3.0.jar) module on gene set collections downloaded from Molecular Signature Database (MSigDB) v7.0 (http://software.broadinstitute.org/gsea/msigdb). GSEAPreranked was applied using the list of all genes expressed, ranked by the fold change, and multiplied by the inverse of the p value with recommended default settings (1000 permutations and a classic scoring scheme). The False Discovery Rate (FDR) was estimated to control the false-positive finding of a given Normalized Enrichment Score (NES) by comparing the tails of the observed and null distributions derived from 1000 gene set permutations. The gene sets with an FDR of less than 0.05 were considered significantly enriched. The RNA-seq data were submitted to the GEO (Gene Expression Omnibus) repository under accession number GSE175894.
Electrophysiology (slice preparation)
Mice were briefly anesthetized with 3% sevoflurane before decapitation. Acute sagittal hippocampal slices (300 μm) were obtained using a VT1200S vibratome (Leica, Switzerland) in ice-cold dissection buffer (212 mM sucrose [Sigma-Aldrich, Cat# S5016], 25 mM NaHCO3 [Sigma-Aldrich, Cat# S6297], 5 mM KCl [Sigma-Aldrich, Cat# P3911], 1.25 mM NaH2PO4 [Sigma-Aldrich, Cat# S0751], 10 mM D-glucose [Sigma-Aldrich, Cat# G7578], 2 mM sodium pyruvate [Sigma-Aldrich, Cat# P2256], 1.2 mM sodium ascorbate [Sigma-Aldrich, Cat# A4034], 3.5 mM MgCl2 [Sigma-Aldrich, Cat# M0250], 0.5 mM CaCl2 [Sigma-Aldrich, Cat# C3881], continuously aerated with 95% O2/5% CO2). The slices were transferred to a chamber filled with warmed (32 °C) artificial cerebrospinal fluid (aCSF: 125 mM NaCl [Sigma-Aldrich, Cat# S7653], 25 mM NaHCO3, 2.5 mM KCl, 1.25 mM NaH2PO4, 10 mM D-glucose, 1.3 mM MgCl2, 2.5 mM CaCl2, aerated with 95% O2/5% CO2) for recovery (30 minutes), and then placed in room temperature prior to the experiments.
Electrophysiology (whole-cell recordings)
Whole-cell recordings were obtained in CA1 hippocampal pyramidal neurons12. In order to avoid confusion, electrophysiology (whole-cell recordings) studies were performed by a non-blinded experimenter. However, recordings were blindly analyzed. Brain slices were transferred to a recording chamber and continuously perfused with aerated aCSF maintained at 25–26 °C. Recordings were made using a MultiClamp 700B amplifier (Molecular Devices, USA) and Digidata 1440 A (Molecular Devices) under visual control (BX50WI, Olympus, Japan). Data were acquired with Clampex 11.0.3 (Molecular Devices) and analyzed using Clampfit 11.0.3 (Molecular Devices) blind to the condition of the mice. Ra (access resistance), Rm (membrane resistance), and Cm (membrane capacitance) were measured using the membrane test protocol included in the Clampex 11.0.3 software. Whole-cell recordings were included only in the following conditions: initial Ra <20 MΩ; Rm >100 MΩ; Cm >100 pF; and <20% change in Ra at the end of the recording.
Intrinsic excitability of hippocampal CA1 cells was recorded using a K-gluconate-based internal solution: 137 mM K-gluconate (Sigma-Aldrich, Cat# P1847), 5 mM KCl (Sigma-Aldrich, Cat# P3911), 10 mM HEPES (Sigma-Aldrich, Cat# H3375), 0.2 mM EGTA (Sigma-Aldrich, Cat# E3889), 10 mM Na-phosphocreatine, 4 mM Mg-adenosine triphosphate (ATP, Sigma-Aldrich, Cat# A9187), and 0.5 mM Na-guanosine triphosphate (GTP, Sigma-Aldrich, Cat# G8877), corrected to pH 7.2, 280 mOsm. To inhibit postsynaptic responses, 100 μM Picrotoxin (Sigma-Aldrich, Cat# P1675), 10 μM NBQX (TOCRIS, Cat# 0373), and 50 μM D-AP5 (Alomone Labs, Cat# D-145) were added to the aCSF. Resting membrane potential (RMP) was measured under ‘I = 0 mode’ for 1 minute after whole-cell configuration. After stabilization, RMP was adjusted to −70 mV and current inputs were increased from 0 to 110 pA (600 ms, 10 pA increase per sweep, 10-s interval). Spontaneous action potentials (sAP) were measured by perfusing standard aCSF, using the same internal solution. Spontaneous firings were detected under ‘I = 0 mode’ for 10 minutes.
The internal solution for recordings of spontaneous excitatory postsynaptic currents (sEPSC) consisted of 117 mM CsMeSO4 (Sigma-Aldrich, Cat# C1426), 10 mM tetraethylammonium chloride (TEA-Cl, Sigma-Aldrich, Cat# T2265), 8 mM NaCl (Sigma-Aldrich, Cat# S9888), 10 mM HEPES (Sigma-Aldrich, Cat# H3375), 5 mM QX314-Cl (TOCRIS, Cat# 2313), 4 mM Mg-adenosine triphosphate (ATP, Sigma-Aldrich, Cat# A9187), 0.3 mM Na-guanosine triphosphate (GTP, Sigma-Aldrich, Cat# G8877), 10 mM EGTA (Sigma-Aldrich, Cat# E3889). For spontaneous inhibitory postsynaptic current (sIPSC) recordings, CsMeSO4 was replaced with 115 mM CsCl (Sigma-Aldrich, Cat# C4036). Neurons were voltage-clamped at −70 mV during the recordings. Picrotoxin (100 µM, Sigma-Aldrich, Cat# P1675) was added for sEPSC recordings, while NBQX (10 µM, TOCRIS, Cat# 0373) and D-AP5 (50 µM, Alomone labs, Cat# D-145) were added during sIPSC recordings. Recording of miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) was separately measured after additionally adding tetrodotoxin (0.5 µM, Abcam, Cat# ab120055).
Statistics and reproducibility
Sample sizes were based on the previous studies12,26,41. For electrophysiological data, the average of each animal (at least 2 cells per animal) was used. Data were analyzed using the R statistical software package (4.0.3; R Core Team, Austria). All continuous variables were tested for normality and homogeneity of variance. ANOVA (One-way analysis of variance) was performed when both conditions were met. Welch’s ANOVA was performed when homogeneity of variance was not met, and the Kruskal–Wallis test was performed if normality was not met. For the CPP test, paired t-test (when normality was met) or Wilcoxon test (when normality was unmet) was used. Repeatedly measured data were such as daily experiments analyzed via Repeated measures analysis of variance (RM-ANOVA) or Friedman rank-sum test. P values < 0.05 were considered significant. Detailed statistic results are described as supplementary data (Supplementary Notes 1–15).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Source data underlying figures are presented in Supplementary Data 2. The RNA-seq data are available from the GEO (Gene Expression Omnibus) repository under accession number GSE175894.
References
Andropoulos, D. B. & Greene, M. F. Anesthesia and developing brains - implications of the FDA warning. N. Engl. J. Med. 376, 905–907 (2017).
Cabrera, O. H., Useinovic, N. & Jevtovic-Todorovic, V. Neonatal anesthesia and dysregulation of the epigenome†. Biol. Reprod. 105, 720–734 (2021).
Chung, W. et al. Sevoflurane exposure during the critical period affects synaptic transmission and mitochondrial respiration but not long-term behavior in mice. Anesthesiology 126, 288–299 (2017).
Zhou, H., Xie, Z., Brambrink, A. M. & Yang, G. Behavioural impairments after exposure of neonatal mice to propofol are accompanied by reductions in neuronal activity in cortical circuitry. Br. J. Anaesth. 126, 1141–1156 (2021).
Yang, Y. et al. Testosterone attenuates sevoflurane-induced tau phosphorylation and cognitive impairment in neonatal male mice. Br. J. Anaesth. 127, 929–941 (2021).
Del Pino, I., Rico, B. & Marin, O. Neural circuit dysfunction in mouse models of neurodevelopmental disorders. Curr. Opin. Neurobiol. 48, 174–182 (2018).
Meredith, R. M. Sensitive and critical periods during neurotypical and aberrant neurodevelopment: a framework for neurodevelopmental disorders. Neurosci. Biobehav. Rev. 50, 180–188 (2015).
Lee, E., Lee, J. & Kim, E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol. Psychiatry 81, 838–847 (2017).
Zhao, T. et al. Prenatal sevoflurane exposure causes neuronal excitatory/inhibitory imbalance in the prefrontal cortex and neurofunctional abnormality in rats. Neurobiol. Dis. 146, 105121 (2020).
Cui, J. et al. General anesthesia during neurodevelopment reduces autistic behavior in adult BTBR mice, a murine model of autism. Front. Cell. Neurosci. 15, 772047 (2021).
Xie, L. et al. Neonatal sevoflurane exposure induces impulsive behavioral deficit through disrupting excitatory neurons in the medial prefrontal cortex in mice. Transl. Psychiatry 10, 202 (2020).
Ju, X. et al. Increasing the interval between repeated anesthetic exposures reduces long-lasting synaptic changes in late post-natal mice. J. Neurochem. 156, 76–87 (2021).
McCann, M. E. et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393, 664–677 (2019).
Sun, L. S. et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 315, 2312–2320 (2016).
Warner, D. O. et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the mayo anesthesia safety in kids (MASK) study. Anesthesiology 129, 89–105 (2018).
Ing, C. et al. Prospectively assessed neurodevelopmental outcomes in studies of anaesthetic neurotoxicity in children: a systematic review and meta-analysis. Br. J. Anaesth. 126, 433–444 (2021).
Shi, Y. et al. Moderators of the association between attention-deficit/hyperactivity disorder and exposure to anaesthesia and surgery in children. Br. J. Anaesth. 127, 722–728 (2021).
DiMaggio, C., Sun, L. S. & Li, G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth. Analg. 113, 1143–1151 (2011).
Walkden, G. J. et al. Early childhood general anesthesia and neurodevelopmental outcomes in the avon longitudinal study of parents and children birth cohort. Anesthesiology 133, 1007–1020 (2020).
Lüscher, C. & Malenka, R. C. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69, 650–663 (2011).
van Huijstee, A. N. & Mansvelder, H. D. Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Front. Cell. Neurosci. 8, 466 (2014).
Ungless, M. A., Whistler, J. L., Malenka, R. C. & Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587 (2001).
Bajo M., Madamba S. G., Roberto M., Siggins G. R. Acute morphine alters GABAergic transmission in the central amygdala during naloxone-precipitated morphine withdrawal: role of cyclic AMP. Front. Integr. Neurosci. 8, 45 (2014).
Kumar, A. & Kohli, A. Comeback of ketamine: resurfacing facts and dispelling myths. Korean J. Anesthesiol. 74, 103–114 (2021).
Sassano-Higgins, S., Baron, D., Juarez, G., Esmaili, N. & Gold, M. A review of ketamine abuse and diversion. Depress Anxiety 33, 718–727 (2016).
Lee, Y. et al. Interval-dependent neurotoxicity after multiple ketamine injections in late postnatal mice. J. Anesth. 35, 93–101 (2021).
De Roo, M. et al. Anesthetics rapidly promote synaptogenesis during a critical period of brain development. PloS One 4, e7043 (2009).
Hitchcock, L. N. & Lattal, K. M. Involvement of the dorsal hippocampus in expression and extinction of cocaine-induced conditioned place preference. Hippocampus 28, 226–238 (2018).
Nam, M. H. et al. Activation of astrocytic μ-opioid receptor causes conditioned place preference. Cell Rep. 28, 1154–1166.e1155 (2019).
Xia, L., Nygard, S. K., Sobczak, G. G., Hourguettes, N. J. & Bruchas, M. R. Dorsal-CA1 hippocampal neuronal ensembles encode nicotine-reward contextual associations. Cell Rep. 19, 2143–2156 (2017).
Byer, D. E. & Gould, A. B. Jr. Development of tolerance to ketamine in an infant undergoing repeated anesthesia. Anesthesiology 54, 255–256 (1981).
Yalçın Çok, O., Evren Eker, H. & Arıboğan, A. Ketamine dosing for sedation during repeated radiotherapy sessions in children. Turk. J. Med. Sci. 48, 851–855 (2018).
Trujillo, K. A., Zamora, J. J. & Warmoth, K. P. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol. Psychiatry 63, 178–183 (2008).
Rocha, A., Hart, N. & Trujillo, K. A. Differences between adolescents and adults in the acute effects of PCP and ketamine and in sensitization following intermittent administration. Pharmacol. Biochem. Behav. 157, 24–34 (2017).
Steketee, J. D. & Kalivas, P. W. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol. Rev. 63, 348–365 (2011).
McKendrick, G. & Graziane, N. M. Drug-induced conditioned place preference and its practical use in substance use disorder research. Front. Behav. Neurosci. 14, 582147 (2020).
Whyte, A. J., Torregrossa, M. M., Barker, J. M. & Gourley, S. L. Editorial: long-term consequences of adolescent drug use: evidence from pre-clinical and clinical models. Front. Behav. Neurosci. 12, 83 (2018).
Parise, E. M. et al. Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol. Psychiatry 74, 750–759 (2013).
Bardo, M. T. & Bevins, R. A. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153, 31–43 (2000).
Hwang, H. & Rhim, H. Acutely elevated O-GlcNAcylation suppresses hippocampal activity by modulating both intrinsic and synaptic excitability factors. Sci. Rep. 9, 7287 (2019).
Garcia-Carachure, I. et al. Enduring effects of adolescent ketamine exposure on cocaine- and sucrose-induced reward in male and female C57BL/6 mice. Neuropsychopharmacology 45, 1536–1544 (2020).
Bates, M. L. S. & Trujillo, K. A. Long-lasting effects of repeated ketamine administration in adult and adolescent rats. Behav. Brain Res. 369, 111928 (2019).
Franco, D. et al. Early-life ketamine exposure attenuates the preference for ethanol in adolescent Sprague-Dawley rats. Behav. Brain Res. 389, 112626 (2020).
Cabrera, O. H., Gulvezan, T., Symmes, B., Quillinan, N. & Jevtovic-Todorovic, V. Sex differences in neurodevelopmental abnormalities caused by early-life anaesthesia exposure: a narrative review. Br. J. Anaesth. 124, e81–e91 (2020).
Carrier, N. & Kabbaj, M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34 (2013).
Franceschelli, A., Sens, J., Herchick, S., Thelen, C. & Pitychoutis, P. M. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience 290, 49–60 (2015).
Dossat, A. M., Wright, K. N., Strong, C. E. & Kabbaj, M. Behavioral and biochemical sensitivity to low doses of ketamine: Influence of estrous cycle in C57BL/6 mice. Neuropharmacology 130, 30–41 (2018).
Strong, C. E. et al. Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology 121, 195–203 (2017).
Thelen, C., Sens, J., Mauch, J., Pandit, R. & Pitychoutis, P. M. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav. Brain Res. 312, 305–312 (2016).
Workman, A. D., Charvet, C. J., Clancy, B., Darlington, R. B. & Finlay, B. L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 33, 7368–7383 (2013).
Charvet, C. J. Closing the gap from transcription to the structural connectome enhances the study of connections in the human brain. Dev. Dyn. 249, 1047–1061 (2020).
Otis, J. M., Fitzgerald, M. K. & Mueller, D. Inhibition of hippocampal β-adrenergic receptors impairs retrieval but not reconsolidation of cocaine-associated memory and prevents subsequent reinstatement. Neuropsychopharmacology 39, 303–310 (2014).
Sakaguchi, M. et al. Inhibiting the activity of CA1 hippocampal neurons prevents the recall of contextual fear memory in inducible ArchT transgenic mice. PloS One 10, e0130163 (2015).
Tuscher, J. J., Taxier, L. R., Fortress, A. M. & Frick, K. M. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol. Learn Mem. 156, 103–116 (2018).
Tannenholz L., Jimenez J. C., Kheirbek M. A. Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Front. Behav. Neurosci. 8, 147 (2014).
Offenhauser, N. et al. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell 127, 213–226 (2006).
Trujillo, K. A. & Heller, C. Y. Ketamine sensitization: Influence of dose, environment, social isolation and treatment interval. Behav. Brain Res. 378, 112271 (2020).
Patil, S. S., Sunyer, B., Hoger, H. & Lubec, G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav. Brain Res. 198, 58–68 (2009).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF), Grant number: NRF- 2017R1A5A2015385, NRF-2018R1C1B6003139, NRF-2019M3E5D1A02068575.
Author information
Authors and Affiliations
Contributions
J.C., X.J. designed, developed, and executed experiments and prepared manuscript; B.H. analyzed the data; Y.L., J.P., W.H.S., H.K. performed experiments; K.H., M.J.L., Y.H.K., Y.K. helped in the design and development of experiments, and in oversight; W.C., J.Y.H. designed, developed, oversaw, and prepared manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Polymnia Georgiou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Christian Wozny and George Inglis. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cui, J., Ju, X., Lee, Y. et al. Repeated ketamine anesthesia during neurodevelopment upregulates hippocampal activity and enhances drug reward in male mice. Commun Biol 5, 709 (2022). https://doi.org/10.1038/s42003-022-03667-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-022-03667-4
- Springer Nature Limited