Abstract
Gene expression toxicity is an important biological phenomenon and a major bottleneck in biotechnology. Escherichia coli BL21(DE3) is the most popular choice for recombinant protein production, and various derivatives have been evolved or engineered to facilitate improved yield and tolerance to toxic genes. However, previous efforts to evolve BL21, such as the Walker strains C41 and C43, resulted only in decreased expression strength of the T7 system. This reveals little about the mechanisms at play and constitutes only marginal progress towards a generally higher producing cell factory. Here, we restrict the solution space for BL21(DE3) to evolve tolerance and isolate a mutant strain Evo21(DE3) with a truncation in the essential RNase E. This suggests that RNA stability plays a central role in gene expression toxicity. The evolved rne truncation is similar to a mutation previously engineered into the commercially available BL21Star(DE3), which challenges the existing assumption that this strain is unsuitable for expressing toxic proteins. We isolated another dominant mutation in a presumed substrate binding site of RNase E that improves protein production further when provided as an auxiliary plasmid. This makes it easy to improve other BL21 variants and points to RNases as prime targets for cell factory optimisation.
Similar content being viewed by others
Introduction
Recombinant production of proteins enables biochemical and structural studies, and the pET vectors hosted in Escherichia coli strain BL21(DE3) are the most popular approach in research laboratories1. Fast growth, high cell density, inexpensive culturing, availability of more than 100 pET expression vectors2 combined with the detailed knowledge of E. coli’s genetics, physiology and metabolism make it the preferred laboratory workhorse.3 This is evident from the more than 200,000 research publications that have cited the use of the pET vectors, and that currently more than 128,000 (86% of the recombinant) structures in the Protein Data Bank and approximately half of the proteins produced worldwide for research and commercial use are produced in E. coli2,4. This remarkable success is despite that bacteria often show impaired growth and fitness-loss when being used for protein production5—a problem that is highly gene-specific, and we still lack clear guiding principles for gene and cell factory optimisation6.
Membrane proteins (MPs) are important drug targets and serve essential roles in basic cellular mechanisms. In both prokaryotes and eukaryotes, 20–30% of all genes encode membrane proteins.7 MPs are involved in fundamental mechanisms such as transport of nutrients and signal molecules, response to environmental changes, membrane stability, maintenance of the redox potential, defence and energy conversion. With natural abundances often too low to isolate sufficient material for in vitro studies, structural and biochemical investigations are limited by our ability to produce and purify MPs recombinantly in a functional state.
MPs are also notoriously known for causing burden in expression systems. They need to be properly inserted into the membrane and cannot be produced in inclusion bodies. This often leads to an overload of the membrane translocation machinery and has previously been reported to severely hamper protein homoeostasis in the cytoplasm leading to the accumulation of aggregates of proteases, chaperones, and overexpressed MPs5,8,9. In addition, competition for the limited space of the membrane causes decreased levels of key respiratory chain complexes and upregulation of the Arc two-component system, indicating serious alterations in central metabolism5.
Experimental evolution has shown great capacity to provide fundamental insights into non-intuitive molecular mechanisms that escape discovery by hypothesis-driven research10. One solution to the gene expression burden is trivial: any mutation that will decrease the expression of the recombinant gene will provide a selective advantage. Previous attempts to evolve BL21(DE3) for better protein production have repeatedly demonstrated this. DE3 strains carry the T7 RNA polymerase (RNAP) gene under the control of the lacUV5 promoter, a stronger version of the native lacZ promoter inducible with IPTG. This allows the expression of any gene of interest under the control of a T7 promoter. Different strains have been isolated in which the toxicity of membrane protein production is reduced, leading to improved production yields: the Walker strains C41(DE3) and C43(DE3)11, evolved in the late 1990s to tolerate overproduction of membrane proteins, the more recently characterised derivative mutant5612 evolved for higher production of the toxic membrane protein YidC, and the strains C44(DE3) and C45(DE3)13 evolved similarly. In all these cases, gene expression induced by IPTG inhibited colony formation on agar plates before mutations occurred and tolerance was achieved mainly due to reduced T7 RNAP activity, either via lacI mutations14, or via promoter modifications, point mutations, or truncations in the T7 RNAP gene.
Here, we aimed at learning more about the gene expression burden, hoping to isolate new types of mutations in the genome of BL21(DE3) by restricting the evolutionary solution space for tolerance on three different levels: we deleted the genomic homology region known to frequently recombine with the λDE3 lysogen in BL21(DE3), we coupled expression of the toxic gene to expression of both green fluorescent protein (GFP) and an antibiotic resistance gene, and we allowed bacterial colonies to form prior to induction of toxic gene expression and isolated mutants over a week-long incubation period.
Results
Restricting the evolutionary space of BL21(DE3) for the production of toxic proteins
In DE3 strains, the strong lacUV5 promoter drives expression of T7 RNAP, and it was previously shown that homologous recombination between lacUV5 and the weaker wild-type lac promoter is a dominating event that within hours of introducing a toxic gene leads to tolerance in BL21(DE3)15. To prevent this, we first genetically restricted BL21(DE3), creating a ∆lacI∆lacZ variant by deleting exactly the part of the native lac locus that shared homology with the λDE3 locus (Fig. 1). In addition, this should prevent the expression of lacY, encoding the lactose permease, allowing a more uniform, concentration-dependent entry of IPTG into all cells in bacterial populations16.
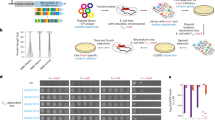
a Illustration showing the experimental set-up used to restrict the evolutionary space for BL21(DE3) to overcome protein production toxicity on three levels: (upper panel) genetic restriction was accomplished by deleting a part of the BL21(DE3) genome that frequently recombines and lowers the T7 RNAP expression, (middle panel) protein production was coupled to both fluorescence and antibiotic resistance to prevent the formation of non-producing mutants and (lower panel) a spatiotemporally structured environment of ageing bacterial colonies was designed in order to identify strong phenotypic mutants. b Schematic of the workflow to produce layered agar plates that allow diffusion of the antibiotic ampicillin (AMP) and the inducer IPTG to allow sufficient time for colony formation on the surface of the top agar layer before toxic protein production is induced.
Next, similar to a previous study12, we chose the E. coli membrane protein insertase YidC coupled to GFP to serve as a model protein to investigate stress caused by membrane protein overproduction as it formerly was shown to have a strong negative fitness effect on E. coli17,18. The GFP fusion comes in handy for phenotypic restriction because it allows visual screening for mutants that still produce the fusion protein. We further restricted the evolutionary solution space by introducing a hairpin structure in the expression plasmid that couples YidC-GFP to the expression of a ß-lactamase gene19, providing resistance to ampicillin (Fig. 1). This way, the formation of non-producing populations should be minimised in the presence of the antibiotic.
Finally, in contrast to the previous studies11,13,20, we aimed at introducing the protein production stress after bacterial colonies had established on plates. This spatiotemporally different approach should allow the formation of a large number of bacteria in a state of dormancy and in a structured environment, which previously was shown to constitute a unique evolutionary environment21. Furthermore, we speculated that mutants would be easy to identify as fluorescent secondary colonies, so-called papillae, outgrowing from the initially established colonies. To this end, two-layered agar plates were poured, allowing slow IPTG and ampicillin diffusion from the bottom to the top layer to grant sufficient time for colony formation before YidC-GFP production was induced (Fig. 1).
During incubation for 1 week at 37 °C, we observed several fluorescent papillae (Fig. 1) that were restreaked to confirm the fluorescent and ampicillin-resistant phenotype. Based on the fluorescence phenotype, the most promising mutants were isolated and cured of the YidC-GFP plasmid using a mild and simple CRISPR-based approach to plasmid curing22. These strains were subsequently retransformed with the original YidC-GFP plasmid to ensure that mutations leading to tolerance were localised on the genome and not on the expression plasmid. A single clone (Evo21(DE3)) was chosen for further characterisation.
Characterisation of Evo21(DE3)’s protein production capabilities
To benchmark Evo21(DE3), we compared its ability to produce the YidC-GFP-fusion protein to the non-evolved BL21(DE3) wild-type strain as well as the derivative Mt56(DE3) previously described to be optimised for YidC-GFP overproduction12. The plasmid pLysS was utilised to limit basal T7 RNAP expression23 in BL21(DE3) and Evo21(DE3). Co-expression of pLysS in Mt56(DE3) is not relevant due to the greatly reduced polymerase activity in this strain. In liquid culture, based on GFP fluorescence, Evo21(DE3) expressed significantly (P ≤ 0.0001) higher amounts of YidC-GFP than BL21(DE3) and Mt56(DE3). Eight hours post induction, yields were 3.6-fold higher in cultures of Evo21(DE3) than in cultures of BL21(DE3) and 2.1-fold higher than in Mt56(DE3) (Fig. 2a). Parallel monitoring of the optical density of the cultures showed no major growth impairment for the strains (Supplementary Fig. 1).
a Production of a toxic YidC-GFP-fusion protein in Evo21(DE3) compared to the non-evolved BL21(DE3) wild-type strain, the BL21(DE3)∆lacI/Z ancestor strain, and a previously evolved BL21(DE3) derivative Mt56(DE3). On the illustrated expression vector (pYidC), YidC-GFP production is translationally coupled to ampicillin resistance. b Fluorescence fold change between Evo21(DE3) and BL21(DE3), producing a library of 24 proteins of the E. coli inner membrane proteome C-terminally fused to GFP. c gfp expression levels in Evo21(DE3) and control strains with and without co-expression of the helper plasmid pLysS. d Western blot showing the expression of a camelid-derived single-chain antibody (nanobody) in Evo21(DE3) and control strains. Samples were normalised to cell density before loading. e Production of YidC under the control of a PrhaBAD promoter allowing titration of expression l-rhamnose. All fluorescence values displayed are normalised to OD at 600 nm. Error bars indicated represent the average squared deviation from the mean (SD) of three biologically independent samples (n = 3).
To assess whether the Evo21(DE3) phenotype was gene-specific, we next investigated the expression of a set of 24 different GFP-fusion proteins selected from an expression vector library of the E. coli inner membrane proteome24. These membrane proteins were selected to cover a wide range of functions, toxicity (previously reported as a change in OD600 upon IPTG addition24), and the number of predicted transmembrane domains (Supplementary Table 1). Comparing the fold change of protein production in Evo21(DE3) and BL21(DE3), titre was improved for 19 of the 24 proteins—with a significant fold change of more than 1.5-fold for 14 of them (Fig. 2b). The highest improvement was 6.1-fold (P ≤ 0.001), observed for the protein YihG.
As a first test that the underlying mechanism allowing Evo21(DE3) to produce more toxic protein was not related to a general decrease in the activity of the T7 expression system—as previously observed for the BL21(DE3) derivatives C41/43(DE3), C44/45(DE3) and Mt56(DE3)—we Sanger sequenced the T7 RNAP gene, which confirmed an absence of mutations. Next, we compared the expression of two non-toxic soluble proteins, GFP and a camelid-derived nanobody, and both were produced at higher levels in Evo21(DE3) than in BL21(DE3) or Mt56(DE3)—both in the presence and absence of pLysS (Fig. 2c, d). Similarly, Evo21(DE3) outperformed other strains in the production of seven out of eight different plant-derived cytochrome P450 enzymes—a class of enzymes of significant biotechnological interest25 (Supplementary Fig. 2). This indicates that the causative mutation in Evo21(DE3) is different from previously isolated BL21(DE3) derivatives and probably does not cause a general decrease in T7 RNAP activity.
Even though the screening for improved protein productivity was performed with the highly efficient T7 system, the ideal mutant strain would be capable of producing more protein independently from the promoter system. To explore if this was the case for Evo21(DE3), we replaced the T7 promoter with the l-rhamnose inducible rhaBAD promoter in the yidC-GFP expression vector, transformed it into Evo21(DE3), and expressed the construct by inducing with different rhamnose concentrations in liquid culture. With concentrations of 5 and 20 mM l-rhamnose, Evo21(DE3) produced significantly (P ≤ 0.0001) more protein than BL21(DE3) and Mt56(DE3) (Fig. 2e).
In summary, this initial characterisation shows that the evolved strain can produce a higher titre of a range of different proteins using a T7-system-independent mechanism.
Whole-genome sequencing and identification of a single causative mutation in Evo21(DE3)
The phenotype of Evo21(DE3) prompted us to sequence the strain using Illumina whole-genome sequencing. Two point mutations—one in the argE and one in the fecB locus (Fig. 3a)—and an insertion of a mobile IS1 element into the rne gene were identified. Upon reintroduction of the argE or fecB point mutations into BL21(DE3) by oligonucleotide-based recombineering, the YidC-GFP overexpression phenotype was not obtained (data not shown), whereas, when reintroducing the truncation of the rne locus into BL21(DE3) and the Evo21(DE3) parental BL21(DE3) ∆lacI∆lacZ strain, the YidC-GFP overexpression tolerance phenotype was nearly identical to Evo21(DE3) (Fig. 3b). This makes it highly likely that the rne IS1 insertion is the main causative mutation in Evo21(DE3).
a Illustration of mutations in the Evo21(DE3) genome compared to the ancestor BL21(DE3). Whole-genome sequencing of mutant strain Evo21(DE3) revealed two point mutations (grey) and a truncation of the rne gene caused by the transposition of a mobile element IS1 into the locus. The deletion of the genomically shared homology sequence in the BL21(DE3) ancestor strain with the λDE3 area (335,401–337,123) is annotated. b Production levels of the toxic YidC-GFP-fusion protein in Evo21(DE3) compared to BL21(DE3), as well as BL21(DE3) and the non-evolved ancestor strain BL21(DE3)∆lacI∆lacZ after reintroducing the rne truncation by recombineering. Error bars indicated represent the average squared deviation from the mean (SD) of three biologically independent samples (n = 3). c Illustration of the E. coli RNA degradosome. N- and C-terminal domain of the membrane-bound essential endonuclease RNase E (blue) and the localisation of associated enzymes PNPase, Rhlb and enolase along the C-terminal non-catalytic scaffolding region are displayed. Mutations of the rne gene in Evo21(DE3), BL21Star(DE3) and rne* gene harboured on pLysS-Max are indicated.
The identified IS1 insertion causes a truncation of the encoded 1061-residue E. coli endoribonuclease RNase E after amino acid 702 and, therefore, a polypeptide lacking the last 359 residues of its C-terminus in Evo21(DE3) (Fig. 3c). RNase E is an essential membrane-associated enzyme involved in the maturation of both ribosomal RNA and tRNA, as well as total mRNA decay, and mediates the assembly of a multi-enzyme complex referred to as the RNA degradosome (Fig. 3c). It has previously been shown that only the N-terminal half (1–529) of RNase E, accommodating the active catalytic domain, is essential for cell growth, and the C-terminal non-catalytic region is mostly disordered and known to function as a scaffold mediating the association of the enzymes PNPase, Rhlb and enolase26,27,28.
Interestingly, a similar truncation of the rne locus, rne131, resulting in an RNase E polypeptide lacking its non-catalytic region (amino acid residues 1–584, Fig. 3c) was isolated in a screen for suppressors of a temperature-sensitive allele of the mukB gene26. A later study showed that in strains such as BL21(DE3), introducing the rne131 truncation caused a bulk stabilisation of mRNA degradation, including mRNA produced by T7 RNAP29. The rne131 truncation was engineered into the commercially available BL21Star(DE3) with the rationale that stabilising bulk mRNA would result in increased protein production. However, following the same rationale, the commercial strain also comes with a note suggesting that it might be unsuitable for overexpression of toxic genes.
We compared the expression of six different genes that we previously found expressed better in Evo21(DE3) than in BL21(DE3) with expression in BL21Star(DE3) and found expression levels to be highly similar between BL21Star(DE3) and Evo21(DE3) (Fig. 4a). This provides an independent confirmation that the phenotype of Evo21(DE3) is caused by the truncation of rne.
a Heterologous production of a variety of non-toxic and toxic GFP-fusion proteins in Evo21(DE3) and BL21Star(DE3), both harbouring different truncations of the rne gene, compared to BL21(DE3). b Expression of the same genes in Evo21(DE3) is compared to expression in BL21(DE3) when co-expressing either the auxiliary plasmid pLysS or pLysS-Max. c Schematic illustration of the plasmid pRNE-GFP designed to report on RNase activity. Promoters controlling rne expression are indicated (P1–4)49,50. The expression level of the rne gene can be monitored in vivo via GFP fluorescence signal. d Exploration of the pRNE-GFP reporter plasmid in BL21(DE3) derivatives. Different levels of RNase activity can be monitored in the strains. e Titration of yidC expression in Evo21(DE3), BL21Star(DE3) and BL21(DE3) via increasing levels of l-rhamnose and its effect on the rne regulon expression reporting RNase activation in the cell during toxic membrane protein production. Upper half: Fluorescence corresponds to YidC-GFP production levels. Lower half: Cells harbour both the rne reporter plasmid (pRNE-GFP) and pPrhaBAD-YidC controlling yidC expression (no GFP fusion). Fluorescence levels correspond to GFP produced under the control of the rne regulon. f Effect of the pLyS-Max auxiliary plasmid on RNase activity. Plasmids pLysS and pLysS-Max are co-expressed along with pRNE-GFP in BL21(DE3) cells. To repress leaky expression of the rhamnose promoter controlling rne* expression on pLysS-Max, 0.4% glucose was added where indicated. Error bars indicated represent the average squared deviation from the mean (SD) of three biologically independent samples (n = 3).
A spontaneously occurring dominant mutation in rne increases protein production when supplied on an auxiliary plasmid
The way Evo21(DE3) was isolated from papillae outgrowing colonies on week-old agar plates, and because dominant rne mutants previously have been observed30, made us speculate that different rne variants could be studied by simple co-expression from a plasmid in the presence of wild-type rne on the genome. To test this idea and to compare different variants of the rne gene at different expression levels, we cloned rne variants in front of the rhaBAD promoter on the pLysS plasmid backbone: full-length rne, the Evo21(DE3) and BL21Star(DE3) truncated versions and a version with a further truncation in the membrane-binding domain of RNase E. However, none of these constructs showed any positive effect on YidC-GFP expression (Supplementary Fig. 3).
Serendipitously, we isolated a spontaneously occurred rne mutant, called rne*, and included it in our analysis. We found that rne* provided on the pLysS plasmid (hereafter called pLysS-Max) could increase YidC-GFP production even further than Evo21(DE3) (Fig. 4b). The rne* mutation converts the essential31 aspartate residue in position 346 to an asparagine in the so-called DNase I subdomain of RNase E involved in chelating an essential Mg2+ ion. The aspartate residue is believed to act as a general base to activate the attacking water essential for the catalytic activity of the enzyme32. The replacement of Asp-346 with the polar amino acid Asn was previously shown to decrease RNA cleavage by about 25-fold32. The effect of expressing rne* was not gene-specific as the effect was preserved for three out of four other tested genes (Fig. 4b). This provides an alternative demonstration that manipulating with rne severely affects recombinant protein production and provides a simple tool, in the form of an auxiliary pLysS-Max plasmid that can be transformed into other strains, to improve protein production titre.
Utilising RNase E autoregulation to build a biosensor of RNase activity in the cell
The positive effect of rne truncations such as rne131 on protein production was previously assumed to be due to the stabilisation of the recombinant mRNA29. However, the observation that the rne truncation in Evo21(DE3) leads to tolerance of toxic gene expression suggests a broader role involving balancing of RNA levels more globally.
Autoregulation allows RNase E to continuously adjust its synthesis to that of its substrates by controlling the degradation rate of its own mRNA33,34. This could work as a biosensor for RNase E activity by fusing the promoter and 5´end of rne with a genetic reporter, as previously demonstrated with lacZ34. To explore RNase E activity in our evolved strains for recombinant protein production, we constructed a similar RNase E biosensor (pRNE-GFP) using GFP as a reporter (Fig. 4c).
We transformed the pRNE-GFP reporter into BL21, BL21(DE3), BL21Star(DE3), Evo21(DE3) and Mt56(DE3) and monitored fluorescence in a microplate reader under conditions similar to the typical protein production scenario. Surprisingly, under these conditions, fluorescence was 14-fold reduced in Evo21(DE3) and sevenfold reduced in BL21Star(DE3) compared to the ancestral BL21(DE3 (Fig. 4d). Given that Evo21(DE3) behaves almost identically to BL21Star(DE3) and that the rne131 truncation has been shown to cause a bulk stabilisation of mRNA degradation29, this suggests that low fluorescence from our reporter correlates with increased bulk mRNA stabilisation. Interestingly, fluorescence from the reporter was reduced approximately twofold in BL21 compared to BL21(DE3), suggesting an effect of the λDE3 locus itself on RNase E activity in the cell.
Next, we wanted to explore if the expression of YidC-GFP affected RNase E activity in the different strains. To this end, because YidC-GFP cannot be expressed from the T7 promoter in BL21 (no T7 RNA polymerase) or BL21(DE3) (no growth), we expressed it from the rhaBAD promoter construct (Fig. 2e) using different concentrations of rhamnose. This showed that YidC-GFP levels could be titrated with rhamnose and confirmed higher expression in BL21Star(DE3) and Evo21(DE3) than in the other strains at high rhamnose concentrations (Fig. 4e, upper half).
To monitor fluorescence from the RNase E GFP reporter, we then deleted GFP from the YidC construct and attempted to co-transform it with pRNE-GFP into different strain backgrounds (Fig. 4e, lower half). However, we were unable to recover and grow transformants in BL21(DE3) and BL21Star(DE3), suggesting a lethal imbalance in RNA levels caused by the presence of these two plasmids. We were able to recover and grow double transformants in BL21, Evo21(DE3), and Mt56(DE3) and monitor fluorescence as an indication of RNase E activity. Fluorescence increased to high levels upon increasing the concentration of rhamnose in BL21 and Mt56(DE3), but fluorescence levels were at least 2.5-fold lower (at 1 mM rhamnose) in Evo21(DE3) and hardly increased upon further rhamnose addition. This suggests that RNase E activity towards bulk mRNA is increased when yidC expression is increased but that the activity is lower in Evo21(DE3) than in the other strains (Fig. 4e).
Finally, we explored the effect of the pLysS-Max auxiliary plasmid (harbouring the mutant rne*) on RNase E activity by co-transforming it along with pRNE-GFP into BL21(DE3). As controls, we included a strain co-transformed with pLysS and pRNE-GFP and BL21(DE3) transformed only with pRNE-GFP. Because we previously observed effects on RNase E activity due to the presence of the λDE3 locus, we repressed leaky expression of T7 RNAP from the lacUV5 promoter by adding glucose to the medium and titrated rne* levels with rhamnose. In the absence of glucose and the presence of pLysS, we observed an increase in fluorescence which was repressed by expression of rne* (Fig. 4f). This shows that the pLysS-Max plasmid can be used to regulate RNase E activity in the cell.
Discussion
Gene toxicity is not restricted to membrane proteins, and the protein burden problem has received attention since the 1950s’35,36. No uniform theory explains the protein burden, but commonly it is observed that ribosomal RNA is degraded, similar to what is observed with nutrient starvation37. A recent study explored the global transcriptomic response to overexpression of a set of 45 different genes in E. coli and found highly gene-specific activation of different host responses such as fear vs greed, metal homoeostasis, respiration, protein folding and amino acid and nucleotide biosynthesis6. Thus, gene overexpression shows many similarities with other types of cellular stress, but the role of RNA degradation in stress responses is not well-understood38.
By restricting the evolutionary solution space of the protein production workhorse BL21(DE3), we have discovered a key role for RNA stability in determining the toxicity and productivity of recombinant protein production in E. coli. This provides an example that engineering the evolution of a microbe can help develop useful traits and discover new mechanisms—even with well-characterised systems such as T7-based expression in BL21(DE3). In contrast to previously evolved BL21(DE3)-derivatives, Evo21(DE3) does not contain mutations in T7 RNAP, and it is therefore also able to produce higher levels of non-toxic proteins such as GFP. The observation that RNA is a key player in gene expression toxicity is in line with recent work demonstrating that expression of mRNA codon variants influences the fitness of E. coli independently of translation39.
The causative mutation in rne in the evolved Evo21(DE3) strain is similar to, but distinct from, the rne mutation engineered into BL21Star(DE3). This is surprising because the main mechanism assumed for BL21Star(DE3) was stabilisation of the recombinant mRNA, and it was therefore thought to be suboptimal for toxic proteins. This belief is at least partly based on observations that T7 RNAP transcripts are unusually RNase E-sensitive40. However, as shown here, the positive effect of an rne truncation appears not to be specific to T7 RNAP transcripts, and BL21Star(DE3) previously did show great capacity to produce a number of different membrane proteins41, and the RNase E inhibitor RraA was identified in a screen for genes whose co-expression could suppress membrane protein-induced toxicity42. Possibly, the stabilisation of mRNA caused by the rne mutations in BL21Star(DE3) and Evo21(DE3), or by overexpression of rraA, is not restricted to the recombinant mRNA but also stabilises other essential mRNAs that are otherwise destabilised by resource allocation to producing the recombinant molecule43. It is interesting to note that RNase E encompasses a membrane-binding domain, and the gene now twice has been implicated in membrane protein production stress. Further studies should examine this possible connection.
The rne* mutation D346N is located in the N-terminal endoribonucleolytic domain of RNase E, in the highly conserved region of the RNase E/RNase G family called high similarity region 2 (HSR2)44,45. The region contains an arginine-rich domain that may contact the RNA45, and removal of the negative charge in position 346 negatively influences the catalytic activity of the enzyme31,32. Similarly, a mutation in RNase G (G341S), only five residues away from D346 in an almost completely conserved region44, was previously shown to be defective in the degradation of a specific mRNA but proficient in processing of 16s rRNA46.
Using a GFP-based biosensor, we monitored RNase E activity in different BL21 variants in the presence or absence of pLysS and the YidC overexpression vector. The biosensor was based on the 5’ region of rne fused to gfp and a highly similar construct was previously shown to decrease expression in response to increased RNase E activity due to destabilisation of the rne part of the mRNA34. However, RNase E substrate recognition is highly complex and different domains coordinate a hierarchy of efficiencies with which different RNA targets are attacked31. The decrease in fluorescence from the pRNE-GFP reporter in BL21Star(DE3) shown here suggests an increase in RNase E activity towards the reporter mRNA, but in the same strain bulk mRNA was previously shown to be stabilised29. Furthermore, the work by Lopez et al. indicated no increase in expression of the rne131 truncated mutant compared to the wild-type rne, which would have been expected if the mutant rne affected autoregulation. Regardless of the underlying mechanism, low fluorescence from the pRNE-GFP reporter was observed here in combination with the truncated versions of rne in Evo21(DE3) and BL21Star(DE3), and upon co-expression of the rne* mutant, and higher fluorescence was observed in response to both the presence of the T7 RNA polymerase in itself and when increasing the expression of a foreign gene like yidC. This supports a central role for RNase E activity in determining recombinant protein production efficiency. The pRNE-GFP RNase biosensor may come in handy as a tool for diagnosing RNase activity in other settings and could facilitate a broader understanding of the role of RNA stability in cell factory engineering.
Methods
Bacterial strains and plasmids
E. coli production strain BL21(DE3)47 (Invitrogen, Waltham, MA, USA; Catalogue number: C600003) was used to generate BL21(DE3)∆lacI∆lacZ (described below) employed here to isolate derivate Evo21(DE3). BL21 (New England Biolabs, Ipswich, MA, USA; Catalogue number: C2530H), BL21(DE3) and Mt56(DE3)12 were used as control strains for experiments benchmarking Evo21(DE3)’s performance. Plasmid pLysS was utilised to limit basal T7 RNAP expression via co-expression of the natural T7 RNAP inhibitor T7 lysozyme that binds the polymerase upon expression and inactivates it.23 All proteins produced in this study are listed in Supplementary Table 1. Membrane proteins were produced as C-terminal GFP-His8 fusion proteins from a pET28a+-derived expression vector48. For the creation of expression vector pET28-yidC-gfp-hp-bla (pYidC), used for the evolution experiment which led to the isolation of Evo21(DE3), a 25 bp hairpin loop sequence (5’-TGACATAGGAGGTCCTCCTATGTCA-3’) for strong translational coupling was inserted downstream of the His-tagged gfp sequence followed by an in-frame ß-lactamase gene (bla) conferring resistance to the antibiotic ampicillin as described in Rennig et al.19. All expression vectors encode a lacI gene under the control of its native lacI promoter to reduce T7 RNAP production prior to IPTG induction in λDE3 strains. Plasmid pLysS was chosen to function as the backbone for co-expression of different rne variants to avoid the burden of having three plasmids present simultaneously in the system. All genes expressed on the pLysS backbone were cloned seamlessly in between lysS terminator and T7Φ3.8 promoter controlling T7lysS expression flanked by additional terminator sequences: T7 terminator (upstream) and rrnc terminator (downstream). RNase E reporter plasmid pRNE-GFP was constructed similar to the previously described vector pEZIO134: The complete rne 5’ UTR (555 bp) comprising promoters P1 to P449,50 and the first 181 codons of the E. coli rne gene were joined in-frame to the second codon of a gfp gene. Cells were grown aerobically at either 37 or 30 °C, and 200 rpm in lysogeny broth (LB) (Difco) supplemented with 50 μg/ml kanamycin, 25 µg/ml chloramphenicol or 100 μg/ml ampicillin depending on the resistance marker of the plasmid used.
Genome engineering
Parental strain BL21(DE3)∆lacI∆lacZ was created using λRED recombineering. BL21(DE3) was transformed with plasmid pSIM1951, harbouring genes for λRED recombination proteins exo, bet and gam under the control of a heat-inducible promoter. Recombineering was performed using a tetA integration cassette flanked by 50-bp long homology sequences to the λDE3 homology area in E. coli BL21(DE3). The tetA integration cassette was subsequently removed via the counter selection on NiCl2, establishing a total deletion of 1,722 bp in genome position 335,401–337,123 verified via sanger sequencing. All mutations identified in Evo21(DE3) via next-generation sequencing were re-engineered using MAGE52 and oligos 1–9 (Supplementary Table 2).
Isolation of Evo21(DE3)
To evolve a new BL21(DE3) derivate with enhanced tolerance towards toxic protein expression, BL21(DE3)∆lacI∆lacZ was transformed with the expression vector pYidC (described above) expressing yidC-gfp translationally coupled to expression of a ß-lactamase encoding gene facilitating ampicillin resistance, alongside with plasmid pLysS. Successful transformants were inoculated into LB medium supplemented with 50 μg/ml kanamycin and 25 µg/mL chloramphenicol and grown overnight at 37 °C and 200 rpm in 5-mL falcon tubes. Production of membrane protein YidC C-terminally fused to GFP allows identification of BL21(DE3)∆lacI∆lacZ-derived mutants efficiently producing YidC in the cytoplasmic membrane upon illumination with UV light. Translational coupling to ampicillin resistance provides an additional layer of selection. Only cells producing the membrane protein fusion protein can grow on a growth medium supplied with the antibiotic. This limits the evolutionary space of arising mutations to ones without a negative impact on production levels.
To allow colony formation prior to membrane protein production, 100 µL of undiluted overnight culture had been plated on layered agar plates. In total, 25 mL LB-agar, containing both 50 μg/ml kanamycin and 25 µg/mL chloramphenicol, was poured on top of a hardened 5 mL LB-agar layer, additionally supplemented with 150 µg/mL ampicillin and 1 mM IPTG. Slow diffusion of IPTG and ampicillin into the upper layer gives cells plated on top sufficient time to form colonies before toxic membrane protein production is induced. Plates were incubated at 37 °C for several days and monitored daily for mutant papillae outgrowing the population. Multiple fluorescent colonies could be isolated during the first week, showing higher fluorescence levels than BL21(DE3) in an initial plate reader experiment screening for GFP fluorescence. One isolate, subsequently named Evo21(DE3), consistently showed high GFP levels and was chosen for further studies. To cure Evo21(DE3) of the yidC-gfp expression vector used during the evolution experiment, the CRISPR-Cas9 based pFREE plasmid curing system was employed22. For all experiments performed thereafter, Evo21(DE3) was always freshly transformed with different vectors.
Plate reader experiments
For growth assays, strains were cultured overnight in 5 mL LB liquid growth medium. Dilutions of 1:50 were grown aerobically for 24 h in 96-well plates at 37 °C and 200 rpm using Gas Permeable Adhesive Seal (Thermo Fisher Scientific, Waltham, MA, USA) to avoid evaporation. In all, 1 mM IPTG was added at OD600 = 0.3. Growth (absorbance at 600 nm) and fluorescence (GFP: excitation at 485 nm, emission at 528 nm) was measured in 20 min intervals while continuous shaking using a Synergy H1 plate reader (BioTek Instruments, Winooski, VT, USA). All measurements were performed in triplicate.
Western blots
Whole-cell lysate analysis via SDS-PAGE and immunoblotting53 1 ODU of cells was lysed using CelLytic B 2× Cell lysis reagent (Sigma-Aldrich, Saint Louis, MO, USA) and run on standard SDS-PAGE using 12% polyacrylamide gels with 5x reducing sample buffer (8 M Urea, 10% SDS). In total, 15 µL of each sample were loaded and run for 30 min at 200 V. SDS-PAGEs were blotted on nitrocellulose membranes using the iBlotTM 1 gel transfer system (Invitrogen, Waltham, MA, USA), and His-tagged nanobody was detected using α-His mouse antibody (05-949, LOT: 3033896, 1:1000, Merck Millipore, Darmstadt, Germany) diluted in 2% skim milk, followed by incubation with a secondary HRP-conjugated rabbit α-mouse IgG (AP160P, 1:10,000, Merck Millipore, Darmstadt, Germany) diluted in TBS-T. Proteins were visualised using the Amersham ECL Prime Western Blotting System (GE Healthcare, Chicago, IL, USA) according to the manufacturer’s instructions and an Amersham gel imaging system.
Next-generation sequencing
DNA purification was performed using DNeasy Blood & Tissue Kit 50 (Qiagen, Hilden, Germany) starting with 1 mL overnight culture according to the manufacturer’s instructions. Isolated DNA was eluted twice using 200 µL 10 mM Tris (pH = 8.5). The genomic libraries were generated using the TruSeq DNA HT Library Prep Kit (Illumina, San Diego, CA, USA). Whole-genome sequencing was performed using the Illumina NextSeq 500 system and sequencing adapters D701 (ATTACTCG) and D501 (TATAGCCT). Data analysis was performed using the breseq software developed by D. Deatherage and J. Barrick54.
Statistics and reproducibility
All experiments were performed in biological triplicate. Error bars and significance values were calculated using the program PRISM. Error bars indicated represent the average squared deviation from the mean (SD). A one-way ANOVA with Dunnett’s multiple comparison test was employed to evaluate differences in expression levels of recombinant protein between Evo21(DE3) and other expression hosts. P values <0.05 were accepted statistically significant. The different significance levels indicated as stars in figures correspond to *P value <0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All whole-genome sequencing data connected to this study has been deposited to the Sequence Read Archive (SRA) and can be accessed via BioProject ID PRJNA741995. All other data will be made available upon reasonable request to the corresponding author. The uncropped and unedited western blot image corresponding to Fig. 2d is included as Supplementary Fig. 4.
References
Rosano, G. L., Morales, E. S. & Ceccarelli, E. A. New tools for recombinant protein production in Escherichia coli: a 5-year update. Protein Sci. 28, 1412–1422 (2019).
Shilling, P. J. et al. Improved designs for PET expression plasmids increase protein production yield in Escherichia coli. Commun. Biol. 3, 1–8 (2020).
Pandey, A., Shin, K., Patterson, R. E., Liu, X. Q. & Rainey, J. K. Current strategies for protein production and purification enabling membrane protein structural biology. Biochem. Cell Biol. 94, 507–527 (2016).
Bill, R. M. Playing catch-up with Escherichia coli: using yeast to increase success rates in recombinant protein production experiments. Front. Microbiol. 5, 1–5 (2014).
Wagner, S. et al. Consequences of membrane protein overexpression in E. coli. Mol. Cell. Proteom. 6, 1527–1550 (2007).
Tan, J. et al. Independent component analysis of E. coli’s transcriptome reveals the cellular processes that respond to heterologous gene expression. Metab. Eng. 61, 360–368 (2020).
Wallin, E. & von Heijne, G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 7, 1029–1038 (2008).
Gubellini, F. et al. Physiological response to membrane protein overexpression in E. coli. Mol. Cell. Proteom. 10, M111.007930 (2011).
Wagner, S. et al. Tuning Escherichia coli for membrane protein overexpression. Proc. Natl Acad. Sci. USA 105, 14371–14376 (2008).
Sandberg, T. E., Salazar, M. J., Weng, L. L., Palsson, B. O. & Feist, A. M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 56, 1–16 (2019).
Miroux, B. & Walker, J. E. Over-production of proteins in Escherichia coli mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260, 289–298 (1996).
Baumgarten, T. et al. Isolation and characterization of the E. coli membrane protein production strain Mutant56(DE3). Sci. Rep. 7, 1–14 (2017).
Angius, F. et al. A novel regulation mechanism of the T7 RNA polymerase based expression system improves overproduction and folding of membrane proteins. Sci. Rep. 8, 1–11 (2018).
Kwon, S. K., Kim, S. K., Lee, D. H. & Kim, J. F. Comparative genomics and experimental evolution of Escherichia coli BL21(DE3) strains reveal the landscape of toxicity escape from membrane protein overproduction. Sci. Rep. 5, 16076 (2015).
Schlegel, S., Genevaux, P. & de Gier, J. W. De-convoluting the genetic adaptations of E. coli C41(DE3) in real time reveals how alleviating protein production stress improves yields. Cell Rep. 10, 1758–1766 (2015).
Marbach, A. & Bettenbrock, K. Lac operon induction in Escherichia coli: systematic comparison of IPTG and TMG induction and influence of the transacetylase LacA. J. Biotechnol. 157, 82–88 (2012).
Dalbey, R. E., Kuhn, A., Zhu, L. & Kiefer, D. The membrane insertase YidC. Biochim. Biophys. Acta - Mol. Cell Res. 1843, 1489–1496 (2014).
Kumazaki, K. et al. Crystal structure of Escherichia coli YidC, a membrane protein chaperone and insertase. Sci. Rep. 4, 1–6 (2014).
Rennig, M. et al. TARSyn: tunable antibiotic resistance devices enabling bacterial synthetic evolution and protein production. ACS Synth. Biol. 7, 432–442 (2018).
Schlegel, S., Genevaux, P. & de Gier, J. W. Isolating Escherichia coli strains for recombinant protein production. Cell. Mol. Life Sci. 74, 891–908 (2017).
Sekowska, A., Wendel, S., Fischer, E. C., Nørholm, M. H. H. & Danchin, A. Generation of mutation hotspots in ageing bacterial colonies. Sci. Rep. 6, 4–10 (2016).
Lauritsen, I., Porse, A., Sommer, M. O. A. & Nørholm, M. H. H. A versatile one-step CRISPR-Cas9 based approach to plasmid-curing. Microb. Cell Fact. 16, 1–10 (2017).
Studier, F. W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J. Mol. Biol. 219, 37–44 (1991).
Daley, D. O. et al. Global topology analysis of the Escherichia coli inner membrane proteome. Sci. 308, 1321–1323 (2005).
Zanger, U. M. & Schwab, M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 138, 103–141 (2013).
Kido, M. et al. RNase E polypeptides lacking a carboxyl-terminal half suppress a MukB mutation in Escherichia coli. J. Bacteriol. 178, 3917–3925 (1996).
Vanzo, N. F. et al. Ribonuclease E organizes the protein interactions in the Escherichia coli RNA degradosome. Genes Dev. 12, 2770–2781 (1998).
Grunberg-Manago, M. Messenger RNA stability and its role in control of gene expression in bacteria and phages. Annu. Rev. Genet. 33, 193–227 (1999).
Lopez, P. J., Marchand, I., Joyce, S. A. & Dreyfus, M. The C-terminal half of RNase E, which organizes the Escherichia coli degradosome, participates in mRNA degradation but not rRNA processing in vivo. Mol. Microbiol. 33, 188–199 (1999).
Briegel, K. J., Baker, A. & Jain, C. Identification and analysis of Escherichia coli ribonuclease E dominant-negative mutants. Genetics 172, 7–15 (2006).
Garrey, S. M. et al. Substrate binding and active site residues in RNases E and G: role of the 5′-sensor. J. Biol. Chem. 284, 31843–31850 (2009).
Callaghan, A. J. et al. Structure of Escherichia coli RNase E catalytic domain and implications for RNA turnover. Nature 437, 1187–1191 (2005).
Sousa, S., Marchand, I. & Dreyfus, M. Autoregulation allows Escherichia coli RNase E to adjust continuously its synthesis to that of its substrates. Mol. Microbiol. 42, 867–878 (2001).
Jain, C. & Belasco, J. G. RNase E autoregulates its synthesis by controlling the degradation rate of its own mRNA in Escherichia coli: unusual sensitivity of the Rne transcript to RNase E activity. Genes Dev. 9, 84–96 (1995).
Kurland, C. G. & Dong, H. Bacterial growth inhibition by overproduction of protein. Mol. Microbiol. 21, 1–4 (1996).
Novick, A. & Weiner, M. Enzyme induction as an all-or-none phenomenon. Proc. Natl Acad. Sci. USA 43, 553–566 (1957).
Dong, H., Nilsson, L. & Kurland, C. G. Gratuitous overexpression of genes in Escherichia coli leads to growth inhibition and ribosome destruction. J. Bacteriol. 177, 1497–1504 10.1128/jb.177.6.1497-1504.1995 (1995).
Vargas-Blanco, D. A. & Shell, S. S. Regulation of mRNA stability during bacterial stress responses. Front. Microbiol. 11 (2020).
Mittal, P., Brindle, J., Stephen, J., Plotkin, J. B. & Kudla, G. Codon usage influences fitness through RNA toxicity. Proc. Natl Acad. Sci. USA 115, 8639–8644 (2018).
Makarova, O. V., Makarov, E. M., Sousa, R. & Dreyfus, M. Transcribing of Escherichia coli genes with mutant T7 RNA polymerases: stability of LacZ mRNA inversely correlates with polymerase speed. Proc. Natl Acad. Sci. USA 92, 12250–12254 (1995).
Deacon, S. E. et al. Reliable scale-up of membrane protein over-expression by bacterial auto-induction: from microwell plates to pilot scale fermentations. Mol. Membr. Biol. 25, 588–598 (2008).
Gialama, D. et al. Development of Escherichia coli strains that withstand membrane protein-induced toxicity and achieve high-level recombinant membrane protein production. ACS Synth. Biol. 6, 284–300 (2016).
Gialama, D., Delivoria, D. C., Michou, M., Giannakopoulou, A. & Skretas, G. Functional requirements for DjlA- and RraA-mediated enhancement of recombinant membrane protein production in the engineered Escherichia coli strains SuptoxD and SuptoxR. J. Mol. Biol. 429, 1800–1816 (2017).
Callaghan, A. J. et al. Quaternary structure and catalytic activity of the Escherichia coli ribonuclease E amino-terminal catalytic domain. Biochemistry 42, 13848–13855 (2003).
Kaberdin, V. R. et al. The endoribonucleolytic N-terminal half of Escherichia coli RNase E is evolutionarily conserved in Synechocystis Sp. and other bacteria but not the C-terminal half, which is sufficient for degradosome assembly. Proc. Natl Acad. Sci. USA 95, 11637–11642 (1998).
Wachi, M., Kaga, N., Umitsuki, G., Clark, D. P. & Nagai, K. A novel RNase G mutant that is defective in degradation of AdhE mRNA but proficient in the processing of 16S rRNA precursor. Biochem. Biophys. Res. Commun. 289, 1301–1306 (2001).
Studier, F. W. & Moffatt, B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189, 113–130 (1986).
Drew, D., Lerch, M., Kunji, E., Slotboom, D. J. & de Gier, J. W. Optimization of membrane protein overexpression and purification using GFP fusions. Nat. Methods 3, 303–313 (2006).
Ow, M. C. et al. RNase E levels in Escherichia coli are controlled by a complex regulatory system that involves transcription of the Rne gene from three promoters. Mol. Microbiol. 43, 159–171 (2002).
Mendoza-Vargas, A. et al. Genome-wide identification of transcription start sites, promoters and transcription factor binding sites in E. coli. PLoS ONE 4, (2009).
Datta, S., Costantino, N. & Court, D. L. A set of recombineering plasmids for Gram-negative bacteria. Gene 379, 109–115 (2006).
Wang, H. H. & Church, G. M. Multiplexed Genome Engineering and Genotyping Methods: Applications for Synthetic Biology and Metabolic Engineering, 1st edn, Vol. 498. (Elsevier Inc., 2011).
Zhang, Z. et al. High-level production of membrane proteins in E. coli BL21(DE3) by omitting the inducer IPTG. Microb. Cell Fact. 14, 1–11 (2015).
Deatherage, D. E. & Barrick, J. E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. in Methods in Molecular Biology (eds Sun, L. & Shou, W.) Vol. 1151, 165–188 (Springer New York, 2014).
Acknowledgements
We thank Prof. Daniel O. Daley for sharing the membrane protein expression vector library. This work was supported by a grant from the Novo Nordisk Foundation NNF17OC0027752 to M.H.H.N.
Author information
Authors and Affiliations
Contributions
S.A.H.H. performed the experiments that led to the creation of this article and analysed and interpreted the data. M.H.H.N. and S.A.H.H. contributed to shaping the idea of the manuscript and the experimental design process and wrote the manuscript. All authors revised, read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests. A patent application has been filed by the Technical University of Denmark with the inventors being M.H.H.N. and S.A.H.H. under European Patent Application No. 21177466.6. The application covers the plasmid pLysS-Max described within this manuscript.
Additional information
Peer review information Communications Biology thanks Sung Ho Yoon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Eve Rogers. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heyde, S.A.H., Nørholm, M.H.H. Tailoring the evolution of BL21(DE3) uncovers a key role for RNA stability in gene expression toxicity. Commun Biol 4, 963 (2021). https://doi.org/10.1038/s42003-021-02493-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02493-4
- Springer Nature Limited
This article is cited by
-
Recombinant multiepitope proteins expressed in Escherichia coli cells and their potential for immunodiagnosis
Microbial Cell Factories (2024)
-
Tips for efficiently maintaining pET expression plasmids
Current Genetics (2023)
-
Current trends in biopharmaceuticals production in Escherichia coli
Biotechnology Letters (2022)
-
Cloning and Expression of Heparinase Gene from a Novel Strain Raoultella NX-TZ-3–15
Applied Biochemistry and Biotechnology (2022)
-
A standardized genome architecture for bacterial synthetic biology (SEGA)
Nature Communications (2021)