Abstract
Cardiovascular diseases (CVDs) are a leading cause of death worldwide. For early diagnosis, intervention and management of CVDs, it is highly desirable to frequently monitor blood pressure (BP), a vital sign closely related to CVDs, during people’s daily life, including sleep time. Towards this end, wearable and cuffless BP extraction methods have been extensively researched in recent years as part of the mobile healthcare initiative. This review focuses on the enabling technologies for wearable and cuffless BP monitoring platforms, covering both the emerging flexible sensor designs and BP extraction algorithms. Based on the signal type, the sensing devices are classified into electrical, optical, and mechanical sensors, and the state-of-the-art material choices, fabrication methods, and performances of each type of sensor are briefly reviewed. In the model part of the review, contemporary algorithmic BP estimation methods for beat-to-beat BP measurements and continuous BP waveform extraction are introduced. Mainstream approaches, such as pulse transit time-based analytical models and machine learning methods, are compared in terms of their input modalities, features, implementation algorithms, and performances. The review sheds light on the interdisciplinary research opportunities to combine the latest innovations in the sensor and signal processing research fields to achieve a new generation of cuffless BP measurement devices with improved wearability, reliability, and accuracy.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Cardiovascular diseases (CVDs) are the leading cause of mortality and disease burden globally1. A report from the European Society of Cardiology states that blood pressure (BP) levels and the risk of stroke or myocardial infarction have a continuous linear relationship2, suggesting that BP is a key indicator of the risk of CVD. Conventionally, BP is measured with a sphygmomanometer with an inflatable cuff; the intra-arterial pressure is equal to the cuff pressure, which can be read with a stethoscope or oscillometer. However, auscultatory or oscillometric measurements are intrinsically obtrusive and temporally discrete methods of BP monitoring; thus, the physiological status of a patient may not be recorded in a timely manner, and vital signals even may be missed because some risk patterns are invisible at rest and only occur sporadically during circulatory system stress. Due to the demand for long-term BP monitoring and the inconvenience of the currently available cuff-based methods, efforts have been made to develop wearable and cuffless BP monitoring systems. These systems typically comprise sensors integrated in wearable devices, such as a wristband or earphone, to collect physiological signals. The signals are then fed in a physiological or machine learning model for BP calculation.
In order to select suitable types of sensor signals for BP estimation, it is important to first understand the origin of each type of signal and its relation with cardiac activities. A cardiac cycle consists of a diastolic phase and a systolic phase. During the diastolic phase, the heart chambers are in relaxation and gradually filled with blood returning from the veins. During the systolic phase, heart muscle contracts to produce sufficient pressure in the left ventricle to open the aortic valve, eject the blood into the aorta and pumps blood to the other organs of the body3. This process repeats periodically, resulting in BP oscillating between diastolic BP (DBP) and systolic BP (SBP). The ejection process causes a pulse wave and recoil; the former spreads pulse information along the ventricular system4 and the latter induces detectable body vibration5. The opening and closing events of the aortic and other valves and related cardiac activities can produce a succession of noteworthy sounds6. In the meantime, blood volume variation within a body segment (such as the chest) causes changes in local electrical conductivity7,8. Behind these mechanical behaviors, electrical activities3 of the heart serve as the stimuli and metronome, and the related electrical signals are transmitted to peripheral sites. Based on these cardiac and cardiovascular (CV) activities, different sensing technologies are designed to capture the BP-related information, such as detecting pulse waves by photoplethysmogram (PPG) and tonoarteriogram (TAG)4, ejection recoil by ballistocardiogram (BCG) and seismocardiogram (SCG)5, heart sound by phonocardiogram (PCG)6, local blood volume variation by ultrasound, stroke volume7 and cardiac output8 by impedance plethysmogram (IPG), and electrical activities of the heart by electrocardiogram (ECG). Numerous mathematical models have been developed to analyze these signals, with different types of models providing different forms of BP estimation output, such as pulse transit time (PTT) based models for beat-to-beat BP estimation and learning algorithms for continuous BP wavefunction extraction.
A number of reviews have recently been published to summarize the advances in flexible sensing devices for CV monitoring4,9, algorithm development10,11,12, system realization13,14,15, and performance evaluation16 for cuffless BP measurements. This review provides a holistic review of these emerging technologies for wearable and cuffless BP monitoring, with the aim of exploring opportunities to merge the merits of device and algorithm development. Figure 1 presents a schematic overview of the key components of a BP measurement event. It can be seen that the measurement mainly relies on a series of critical steps, i.e., signal collection from the sensor end and signal processing and analysis at the back-end calculation units. Accordingly, this review includes flexible sensing technologies for BP monitoring, signals from non-flexible sensors, noise reduction, and estimation models for both the beat-to-beat and waveform BP extraction. Finally, a brief summary of the technology development is provided and the future research directions are discussed.
Flexible sensing technologies for BP monitoring
TAG sensors
TAG is a continuous arterial BP-related signal recorded by a pressure sensor or estimated by other cuffless measuring techniques17. Flexible TAG sensors are devices that can convert pressure inputs into electrical outputs. Compared with traditional MEMS-based rigid sensors, the most crucial advantage of flexible TAG sensors is their excellent conformability to curved or soft surfaces, such as human skin18,19,20. Due to this advantage, they can be used as wearable sensors for monitoring key physiological signals, such as epidermal pulses. The BP on the vascular wall of arteries distributed in superficial tissues can be transmitted to the skin surface (Fig. 2a)21. A TAG sensor adhering to the skin surface can thus capture the tiny pressure variations caused by the pulse wave (Fig. 2b)22. This cuffless method can realize continuous pulse monitoring with little user discomfort. A typical pulse waveform comprises three characteristic peaks: the percussion wave (P-wave), tidal wave (T-wave), and diastolic wave (D-wave), as shown in Fig. 2c21. The P-wave is caused by the initial systolic spike due to blood ejection from the contracting left ventricle. The T-wave and D-wave are caused by blood flow reflection from the upper and lower body, respectively23.
a Pulse sensing mechanism. Reprinted with permission from ref. 21. Copyright 2020 Wiley. b A piezoresistive sensor adhering to the human wrist seamlessly. Reprinted with permission from ref. 22. Copyright 2017 Wiley. c A typical pulse waveform consisting of three characteristic peaks. Reprinted with permission from ref. 21. Copyright 2020 Wiley. d Piezocapacitive sensing mechanism. e A piezocapacitive sensor made of a micropillar dielectric layer, and its pressure transfer curve. Reprinted with permission from ref. 24. Copyright 2019 American Chemical Society. f Piezoresistive sensing mechanism. g A piezoresistive sensor made of a carbon-decorated fabric and its pressure transfer curve. Reprinted with permission from ref. 25. Copyright 2015 Wiley. h Piezoelectric sensing mechanism. i A piezoelectric sensor made of a PZT film and its transfer curve. Reprinted with permission from ref. 26. Copyright 2017 Wiley. j Triboelectric sensing mechanism. k A triboelectric sensor made of woven PTFE stripes and a PET film, together with its sensing mechanism. Reprinted with permission from ref. 27. Copyright 2018 Wiley.
Four pressure sensing mechanisms are commonly used: piezocapacitive (Fig. 2d), piezoresistive (Fig. 2f), piezoelectric (Fig. 2h), and triboelectric (Fig. 2j) mechanisms. Piezocapacitive and piezoresistive materials indicate pressure exerted on the material through changes in capacitance (Fig. 2e) and resistance (Fig. 2g)24,25, respectively. Piezoelectric and triboelectric methods transduce pressure into a change in electric potential (Fig. 2i, k) and are thus self-powered devices26,27. All TAG sensors have three essential components: sensing materials, flexible substrates, and electrodes. Flexible substrates are typically made using thin films of polyimide (PI)28,29, polydimethylsiloxane (PDMS)30, and polyethylene terephthalate (PET)31. Flexible electrodes are usually thin films made of conductive metals32 and metal oxides (e.g., indium tin oxide (ITO))33. Sensing materials vary between sensor types and have been discussed in depth in several review articles34,35,36. The general advantages and disadvantages of sensor types are summarized in Table 1. The sensitivity of triboelectric and piezoelectric sensors differs greatly from other sensors; thus, they can only be compared with each other.
ECG and bioimpedance electrodes
An ECG signal is a record of the potential difference between conductive electrodes across the heart. These electrodes (Fig. 3) are attached to the skin of the arms, legs, and chest. Three types of ECG electrodes are used, namely wet37,38,39, dry, and capacitively coupled electrodes40. Wet and dry electrodes are directly connected to the skin and can measure skin impedance via conductive material. By contrast, capacitively coupled electrodes are attached to the skin with an insulating layer, and they measure the capacitively coupled charge with local electronics. Wet electrodes, such as the clinically used Ag/AgCl electrode, can provide a high-quality signal; however, they require electrolytes that may cause skin irritation. This electrolyte also decays over time, requiring replacement. Thus, wet electrodes are unsuitable for long-time ECG monitoring. By contrast, dry electrodes can be used for long-term ECG monitoring; however, the signal quality is comparatively poor due to the high impedance of the skin. Capacitively coupled electrodes with integrated electronics can provide high-quality measurements; however, the cost of fabricating these electrodes is high. The ability to maintain morphological waveforms under both dry and water-immersed conditions is essential for daily ECG monitoring41. In 2018, Ramasamy and Balan42 summarized the performances and fabrication methods for these three types of ECG electrodes. In 2020, Gandhi and Raghava43 reviewed the fabrication methods in a targeted manner. The materials for ECG electrodes should be conductive, biocompatible, and time-durable. Details regarding the conductive materials are listed in Table 2.
Electrodes for ECG can also be used for bioimpedance monitoring, such as IPG monitoring44. IPG, also known as bioimpedance plethysmography (BPG)45, can provide useful features for BP calculation algorithms46,47. For instance, impedance cardiogram (ICG), a special IPG waveform that measures the thoracic electrical bioimpedance, is often used to calculate the pre-ejection period. In bioimpedance monitoring, two pairs of electrodes are required: one for AC current injection (some methods use a short current pulse47) and the other for voltage difference sensing44. In IPG, the electrode placement is flexible; that is, the electrode pairs need not cross the heart as ECG electrodes do. For example, IPG electrodes can be placed on the neck44, wrist48,49, arm7, leg50, feet51, or on multiple body sites45.
Optical sensors
PPG devices comprise light sources and photodetectors. The light sources are typically light-emitting diodes (LEDs), and the photodetectors convert light into PPG signals (Fig. 4a, b). A PPG signal from a photodetector indicates the amount of optical absorption or reflection, which is responsive to the change of blood volume in the optical path. In practice, PPG sensors are routinely placed at peripheral body sites, such as the finger, earlobe, or forehead, to monitor cardiac-induced changes in microvascular blood volume52. Light of ~510–590 nm in wavelength is the primary contributor to the pulsatile component of the reflected light, and infrared light has the greatest penetration depth. Thus, green (565 nm) or yellow (590 nm) light is typically used in reflective PPG sensors, whereas red (680 nm) or near-infrared (810 nm) light is used in transmissive PPG devices. Multiwavelength PPG (MWPPG) can also be used to calculate BP with PTT53.
a Reflective mode. b transmitting mode. c example of PPG signal. d Reflective mode epidermal and transmissive mode flexible hybrid organic/inorganic NIR PPG for BP monitoring. Reprinted with permission from ref. 59. Copyright 2017 Wiley.
Wearable PPG devices, such as eyeglasses54 and bulky pulse oximeters55, have been commercialized for CV monitoring. Scientists have also developed state-of-the-art conformal and unobtrusive PPG sensors with new materials and designs. In 2018, Lee et al.56 designed a ring-like organic LED (OLED) and organic photodiode (OPD) patch that maximized PPG signals in the reflective mode for use as a pulse oximeter. Vital sign tracking can even be achieved under ambient light without LED sources57,58. In 2019, Polat et al.57 successfully demonstrated flexible and transparent wearables based on graphene sensitized with semiconducting quantum dots in a phototransistor (PT) structure. They achieved heart rate and arterial blood oxygen saturation monitoring under ambient light. In 2020, Han et al.58 also produced a pulse oximeter by adding passive optical filters to OPDs. However, estimating BP by applying only PPG techniques is not yet mature; integrating other physiological signals enhances the accuracy of these methods. In 2017, Xu et al.59 combined ECG signals with a flexible organic-inorganic hybrid near-infrared PPG sensor integrating a low-power, high-sensitivity organic PT and a high-efficiency inorganic LED for real-time heart rate variability and BP tracking in both the reflective and transmissive modes (Fig. 4d). Future device optimizations enabling high-precision monitoring of pulse waves, along with improved algorithms, could spur the adoption of PPG for CV monitoring, including for BP estimation60.
Ultrasound sensors
Ultrasound sensors for wall tracking are a promising, noninvasive device for BP waveform monitoring and have increasingly attracted the attention of researchers61. Compared with other noninvasive methods, such as PPG and TAG, the ultrasound wall-tracking technique has a higher penetrating capability, which can be used to track the BP waveform of vasculature embedded in deep tissues62. However, current commercial ultrasound probes are heavy, rigid, and bulky; moreover, the ultrasound probe must be held and stabilized by an experienced operator to achieve a reliable acoustic coupling interface63,64. These problems inevitably lead to inaccurate results because the probe compresses local vasculatures and changes their distension behavior. Therefore, conventional ultrasound probes are unsuitable for the long-term monitoring of BP waveforms.
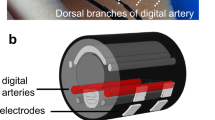
Flexible wearable devices with mechanical properties similar to those of skin enable long-term, continuous, and timely monitoring of vital signs65,66,67. Ultrasound sensors that are flexible and wearable are attractive solutions to the aforementioned problems in ultrasound-based methods. In 2022, Wang et al.68 reported a bioadhesive ultrasound (BAUS) device that utilizes a couplant consisting of a soft yet tough hydrogel covered by a thin antidehydrating elastomer to achieve strong adhesion between the ultrasound probe and skin, thus allowing for long-term continuous imaging and BP tracking. In 2021, Sempionatto et al.69 proposed a noninvasive skin-worn ultrasound sensor for monitoring BP and heart rate. This ultrasound sensor contained 1 × 8 ultrasonic transducers with a kerf of 0.2 mm; each transducer had a size of 4 mm × 1 mm × 0.25 mm. Styrene-ethylene-butylene-styrene (SEBS) block copolymer was used as the substrate, superstrate, and filler. Stretchable electrodes were produced with silver ink and could be integrated in the SEBS substrate. Each ultrasonic transducer vibrated in thickness mode at a fixed frequency of 7 MHz. The sensor should be located over the targeted vessel to measure the BP waveform. To easily place the sensor at the correct position, in 2018, a flexible ultrasound sensor with a 4 × 5 transducer array (f = 7.5 MHz) was developed by Wang et al.70 (Fig. 5). An “island–bridge” layout with a serpentine electrode, produced by stacked polyimide and Cu in a bilayer fashion, was exploited to produce a stretchable device. The transducers could individually generate and receive ultrasound waves. The array design enables sensing and monitoring by a transducer located over the targeted vessel without manual positioning. To protect the device from possible corrosion by sweat, the transducers were encapsulated with silicone; this encapsulation can also be used to produce a conformal device that makes excellent contact with the skin surface.
Reprinted with permission from ref. 70. Copyright 2018 Nature.
Little research has been performed on flexible and wearable ultrasound sensors for monitoring BP waveforms. Integrating the back-end functions of the devices, such as electronic control, signal processing, and power sources, in a stretchable and wearable ultrasound sensor remains challenging. Thus, their practical applications are limited, especially for the long-term and continuous monitoring of BP waveforms. Moreover, although ultrasound sensor arrays with small transducers and small pitches have high measurement accuracy, these sensors are difficult to fabricate with existing technologies71.
Other signals from non-flexible sensors
Apart from the biosignals that have already been measured with flexible sensors, there are other cardiac activity-induced signals that can readily be captured by portable, wearable, or furniture-integrated sensors and may be used to enhance or expand the BP monitoring technologies. For instance, BCG/SCG can indicate CVD-induced slow and longitudinal changes in cardiac functions5 and therefore used to trigger recalibration of BP models. It has been demonstrated that BCG/SCG signals can be obtained by weighing scales, bed-based72, and chair-based devices, as well as accelerometer-embedded wearable devices5. PCG records heart sounds produced by cardiac activities, such as the opening and closing movements of the mitral, tricuspid, aortic, and pulmonary valves, and some key points, such as S1 and S2, can be used for BP estimation6,73.
Noise reduction techniques
Power-line interference (PLI), external electromagnetic (EM) interference, baseline wander (BW), and motion artifacts (MAs) are the most common sources of noise in biosignals5,8,13,74,75,76. PLI superposes 50/60 Hz sinusoidal noise and its higher harmonics associated with the frequency of power lines77 on almost all types of signals. EM noise often appears in ECG and PPG signals but can be removed or reduced through shielding. BW refers to the shift of signal baseline due, for example, to the environment or intrinsic property change of the sensor unit, and normally occurs in a low-frequency band. MAs are signal noise induced by respiration, muscle movements, poor skin-sensor/electrode contact, etc.45,75,78. Noise reduction is an indispensable component for wearable cuffless BP monitoring systems. The most broadly used denoising tool is filter, which has been typically applied to suppress the noise of a certain frequency range in TAG75, ECG40,78,79, IPG7,44,49, PPG13,75, ultrasound76,80, and BCG72,81 signals. While filtering can serve as a universal tool for almost all types of sensing modalities, there have also been signal-specific denoise methods developed to improve the noise removal efficiency and accuracy. Satija et al.74 proposed an automated ECG noise detection and classification system for unsupervised ECG analysis, during which a decision rule-based algorithm is executed to detect the presence of noises and classify input ECG signals into six groups, namely noise-free ECG, ECG + BW, ECG + MA, ECG + PLI, ECG + BW + PLI, and ECG + BW + MA for more accurate denoise treatment. The method achieved average sensitivity, positive predictivity, and classification accuracy of 98.93%, 98.39%, and 97.38% on a large collection of ECG signals taken from five standard databases. Poh et al.82 designed a PPG sensing system with adaptive noise cancellation (ANC) which employed an embedded accelerometer as the reference signal sensor to remove MAs and achieved significantly improved heart rate tracking performances. Ibrahim and Jafari83 developed a generalized extreme studentized deviate (GESD) algorithm that exploits the inter-channel variance of four-channel IPG signals extracted in small areas to remove MAs. Pashaei et al.80 proposed a pre-amplifier design in a flexible ultrasound probe circuit to maintain the signal-to-noise ratio (SNR) during signal transmission from the probe to the receiver, thus enhancing the detection sensitivity to small organs, e.g., blood vessels. ~5 dB reduction in noise figure was visible around the nominal imaging frequency of 5 MHz (transducer resonance) when comparing simulation performance of the proposed active probe with the passive probe. There have also been several noise reduction strategies for BCG/SCG measurements, which are summarized in a recent review by Inan et al.5.
Beat-to-beat BP estimation models
BP estimation techniques are divided into three categories in accordance with the temporal resolution of the output BP information: snapshot, beat-to-beat, and waveform continuous techniques (Fig. 6). Conventional cuff-based methods, such as auscultatory or oscillometric measurement, are used to measure snapshot BP. Thus, beat-to-beat and waveform continuous methods, as in wearable and cuffless devices, are reviewed in this section. Beat-to-beat models provide an estimation of BP parameters at each heartbeat, whereas the continuous models output continuous BP waveforms.
PTT-based methods
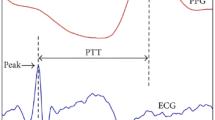
The PTT-based analytical model is a dominant beat-to-beat BP estimation method (Table 3). There are two mainstream definitions of PTT and pulse arrival time (PAT), as illustrated in Fig. 7. Mukkamala et al.84 suggest that PTT is the time period for the pressure wave to travel between two arterial sites and PAT is the time delay between the ECG waveform and its adjacent distal arterial waveform. Meanwhile, Ding et al.85, on the basis of the original meaning of “transit” and the history of relevant research, suggest that PTT should be the time it takes the pulse signal to carry the pulse wave information from one location to another in a CV system, while that PAT refers to the pulse in the same form of energy from the starting point to the arrival point; accordingly, PTT is marked as ①, ②, and ③, while ③ also refers to PAT, as shown in Fig. 7. Moreover, signals used to calculate PTT can be labeled in the subscript to avoid definition ambiguity. With a proper definition, the pre-ejection period (PEP) can be either included in or excluded from PTT85. The Moens–Korteweg equation86 \({PWV}=\sqrt{\frac{E{h}_{0}}{2\rho {R}_{0}}}\) and Hughes equation87 \(E={E}_{0}\exp \left(\xi P\right)\) are fundamental to PTT analysis; \({PWV}\), \(E\), \({h}_{0}\), \(\rho\), and \({R}_{0}\) are pulse wave velocity, the elastic modulus at pressure \(P\), artery thickness, blood density, and artery radius; \({E}_{0}\) and \(\xi\) are the elastic modulus at zero pressure and the material coefficient of the artery. The underlying physiological model assumes that the artery stiffens as the BP increases (Hughes equation), leading to an increase in PWV or a decrease in PTT (M-K equation). In the following, we summarize the typical signal combinations used for BP model implementation.
(1) ECG + PPG: Dating back to 1978, Obrist et al.88 evaluated 114 human subjects and reported that the PTT varied consistently with SBP but not DBP. In the 2000s, Chen et al.89 demonstrated that changes in PTT are linearly related to changes in SBP over a short period. Based on this high-frequency variation, a long-term estimation can be achieved by including a low-frequency baseline extracted from intermittent BP measurements. Poon and Zhang90 developed a technique for simultaneously estimating DBP and SBP. These results suggest that cuffless methods are promising for wearable healthcare. A 6-month study conducted by Wong et al.91 suggested that PTT-based techniques require an auxiliary module or an independent subalgorithm for intermittently tracking baseline changes. In the 2010s, Gesche et al.92 constructed a nonlinear model to describe the relation between PWV and BP and proposed a one-point calibration scheme for the model. The results indicated that the PWV-based estimation method has the potential for BP monitoring; however, customized individual corrections are required due to subject differences. Ding et al.93 proposed an indicator called photoplethysmography intensity ratio (PIR), which takes vasomotor tone into account. The inclusion of PIR in PTT-based BP estimation improved its accuracy93.
(2) MWPPG: On the basis of conventional PPG signals and PTT-based BP estimation methods, Liu et al.53 developed an MWPPG sensor and analyzed its mechanism in 2016. By using the signals extracted with these MWPPG sensors, Liu et al.94 proposed a depth-resolved approach for continuous BP monitoring in 2018.
(3) IPG-based PTT: In 2018, Huynh et al.95 proposed and examined a miniaturized bioimpedance device consisting of two sets of four-electrode interfaces attached around the wrist of a subject to measure IPG-based PTT in a 0.5 × 1.75 cm2 area. Leveraging both the PPG and IPG signals, they included changes in the cross-sectional area of blood vessels in a PTT-based model and improved the BP estimation accuracy96. It is worth noting that BP can also be estimated using IPG-ECG51, IPG-PPG49,97, IPG-IPG8 (including IPG + ICG45) combinations, or even single-channel IPG48. Several studies have evaluated the performance of PTT-based BP estimates using IPG + ECG and PPG + ECG and reported that the former is superior to the latter in a manner7,44.
(4) BCG-based PTT: In 2015, He et al.98 employed a wearable device to monitor BCG, ECG, and PPG simultaneously and avoid interference from PEP by using BCG and PPG to determine PTT. Kim et al.81 showed that BCG can serve as a proximal timing reference for PTT calculation, which may outperform the ECG-based PAT for BP estimation.
(5) TAG + PPG: In 2022, Samartkit et al.75 integrated PPG and TAG to monitor BP and heart rate. The TAG signal was extracted by a lead zirconate titanate (PZT) piezoelectric sensor. In contrast to general PTT calculation, where only the time interval between two adjacent systolic peaks is used, their modified PTT technique measures PTTs for SBP and DBP separately.
It is worth noting that a recent report from Microsoft Research compared different cuffless BP extraction models over more than 1000 subjects and found that the commonly used PAT and pulse wave (PWA) analysis methods do not offer meaningfully more accurate estimation results than those obtained from baseline models99. This finding may reflect the fundamental limitation of the PTT/PWA methods in relying on overly simplified blood vessel-regulated models and calls for developing more comprehensive cuffless BP measurement models with strong theoretical underpinnings100.
Machine learning-based methods
Machine learning (ML) is a powerful tool for algorithmic beat-to-beat BP estimation. ML-based BP estimation methods are categorized into traditional and deep learning (DL) algorithms (Fig. 8). In contrast to traditional ML algorithms, DL methods can perform end-to-end predictions and have a layered model structure with greater depth. The common ML methods, such as linear regression (LR), support vector machine (SVM), k-nearest neighbors (KNN), decision tree (DT), and random forest (RF), are discussed in this review. DL methods include convolutional neural networks (CNN), recurrent neural networks (RNN), and their hybrids.
Traditional ML methods
In contrast with numerous hidden layers and the strong fitting ability of DL, traditional ML models have better interpretability and generalization. Typically, the first step in training a traditional ML model is handcrafted feature extraction for later analysis. Some commonly used features for BP estimation are listed in Table 4.
LR is the most frequently used traditional ML algorithm in BP estimation. Some common optimization methods for LR include least-square and maximum-likelihood estimation. In 2022, Natarajan et al.101 integrated PTT features with PPG data extracted from the finger, ear, and toe to train LR models and eliminated errors in BP prediction. In 2022, Figini et al.102 used PTT and heart rate as features to train several regression algorithms, including LR, for predicting SBP and DBP. In 2021, Mamun103 applied a penalty-based regression outperforming the conventional LR in both SBP and DBP prediction in terms of the mean absolute error (MAE). In 2021, Liu et al.104 evaluated multiple LR models with data from a maximal exercise stress test to achieve a robust cuffless prediction of BP during exercise.
SVM is a powerful and widely used ML method. In SVM, a model is optimized by maximizing the margin between different subspaces in the feature space to achieve accurate classification. SVM models can be used to predict BP with individual-level data104,105 or public datasets106,107. In 2017, Zhang et al.108 applied SVM to classify clean and noise-polluted ECG cycles. Plug-and-play SVM modules are easily available and can accelerate the deployment of BP estimation devices109.
The KNN algorithm differs from other ML methods in that it is a nonparametric supervised learning method. In 2019, Chen et al.110 compared the measurement performance of five models and reported that the KNN model achieved the best result on the MIMIC II physiological database. In 2021, Fati et al.77 embedded a KNN submodel into an automated optimization tool to avoid bias potentially resulting from using a single KNN algorithm. In 2018, Fang et al.111 recovered a PPG signal from a remote PPG monitor and leveraged KNN to predict BP parameters.
DT is constructed by choosing a feature in each iteration step to split the sample set best. The metrics used to indicate the “best” split differ on a case-by-case basis and may include information gain. RF algorithms feature the use of multiple DTs. In 2020, Nath and Thapliyal112 trained a DT regressor and reported that it did not perform as well as an AdaBoost version. In 2022, Gupta et al.113 investigated the prediction accuracy of RF and DT on the UCI and MIMIC I datasets and reported that RF outperformed other models. Farki et al.114 developed a clustering-based algorithm to elevate the performance of BP estimation using RF. In 2021, Ma et al.115 leveraged the information entropy of signals from wearable devices to train an RF model that had higher accuracy than LR or SVR.
DL methods
In CNNs, convolutional kernels are used in the hierarchical architecture of layers to extract spatial and temporal features from data. Although CNN kernels typically have two or more dimensions, CNN kernels used in BP prediction are often one-dimensional because both the input and output signals are temporal sequences. Predictions based on a single modality, such as only PPG, are concise but lack information that could be provided by other modalities. Many researchers have used CNN-based algorithms for multimodal inference using ECG and PPG116,117 or ECG and TAG118. Other auxiliary techniques, such as squeeze and excitation (SE)119, can also improve the performance of CNNs.
RNNs are neural networks that have a “memory” that is consulted while processing each input. Numerous input modalities have been selected for RNNs; researchers have both used a single modality, such as ECG120,121 or PPG122,123, or a combination of modalities124,125,126. To determine the dynamic significance of each data point in a sequence, the attention mechanism, which is common in the field of natural language processing, has been employed in RNN-based BP estimation methods120,122. Typically, information in a neural network is connected between neighboring layers; however, in networks with residual or dense connections, information can “jump” from a node to a node several layers away from it124.
Hybrid models that simultaneously leverage the strengths of various learning algorithms have been created and deployed. In particular, CNNs and RNNs127,128,129,130,131 are frequently combined because these networks can concurrently extract short- and long-term dependencies. The input modality for hybrid models can be single128,130,131,132 or multiple127,129,133. The attention mechanism has also been used in hybrid models to capture the contextual meaning of data points128,129. Because individual-level data are often insufficient and DL methods are compute-intensive, finetuning132 and transfer learning134,135 have been used to increase the performance of methods applied in a new domain. Moreover, physical laws regarding BP or other parameters, such as hemodynamics, can be embedded into the design of an algorithm136.
BP waveform estimation models
In a continuous BP estimation method, the blood pressure waveform is acquired. Because numerous temporally varying details are embedded in a waveform, continuous BP estimation can potentially give doctors more information for diagnosis than beat-to-beat methods can. BP waveform analysis enables inference regarding stroke volume, cardiac output, vascular resistance, and other BP parameters137.
DL-based methods
DL algorithms can fit hidden relationships between input and output data. Thus, DL is applicable in BP estimation for physiological signal inputs extracted from flexible sensors. Due to the versatility of DL algorithms, they can be designed for both beat-to-beat and continuous estimation. In contrast to other nonlearning methods, DL has many intrinsic advantages for continuous BP waveform estimation:
-
Almost all functions, regardless of linearity, can be fitted by a well-designed DL algorithm.
-
The scale of BP-related medical data is enormous. Compared with analytical models, DL has a greater ability to process data concurrently and is thus suitable for computation-intensive tasks.
-
Thanks to the rapid development of algorithmic and computational technologies and the maturity of DL techniques, the ideas and algorithms in this field are amenable to BP waveform prediction.
-
DL can automatically extract features from data; nonlearning methods rely on the bespoke features and may inadvertently neglect critical information, resulting in bias.
CNN networks excel at local feature detection and thus are often used for continuous BP estimation. U-Net, a variant of the CNN framework targeted at biomedical image segmentation, has been adapted to ABP estimation with a raw PPG signal as its input138. Its adaptation to the one-dimensional time domain, named Wave-U-Net, was used by Cheng et al.139 to predict ABP waveforms in 2021. The autoencoder in the network could suppress noise while maintaining the key information. In 2020, Sadrawi et al.140 applied a deep convolutional autoencoder that was optimized with a genetic algorithm to estimate BP using PPG. In 2021, Qin et al.141 merged a convolution-based deep autoencoder with multidomain adversarial training to learn individual differences between subjects. In 2021, Hill et al.142 adapted the V-Net architecture, which was originally designed for image segmentation, for one-dimensional ABP waveform prediction.
In contrast to CNNs, RNNs are frequently used to extract long-term dependencies from data in the time dimension. In 2021, Aguirre et al.143 used gated recurrent units (GRUs) to construct an RNN model to assess BP morphology. Harfiya et al.144 proposed a long short-term memory (LSTM) model for learning signal-to-signal translation between PPG to ABP. Sideris et al.145 tested an LSTM-based RNN and compared it with LR, which is commonly used in beat-to-beat BP estimation.
Mechanics-based methods
The quantitative relation between BP and the cross-sectional area of the blood vessel allows for the automated tracking of the continuous BP waveform. The dominant modality used for extracting blood vessel diameter is ultrasound. An ultrasound transducer can transform sinusoidal or pulse voltage signals into mechanical ultrasound waves76,146. When the ultrasound wave reaches the interfaces between the tissue and the vessel, both transmission and reflection occur. The reflected wave carries critical location information about these interfaces. The ultrasound wave is reflected at both the anterior and posterior interfaces between the tissue and the vessel, enabling changes in vessel diameter over time to be recorded based on the received ultrasound signals80,147. The measured diameter change of the vessel can be translated into localized BP waveforms according to the physical model of vessel148. BP can be calculated as
where \({p}_{d}\) is the diastolic pressure, \({A}_{d}\) is the cross-section of the vessel during cardiac diastole, \(\alpha\) is the vessel rigidity coefficient, and \(A\left(t\right)\) is the cross-section of the vessel over time. If the vessel is assumed to be rotationally symmetrical, \(A\left(t\right)\) can be expressed as \(\frac{1}{4}\pi {d}^{2}\left(t\right)\), where d(t) is the measured diameter waveform of the target vessel70.
Calibration
Most of the aforementioned models, in particular the PTT and pulse wave analysis (PWA) based methods, require a calibration process where a few data points or a segment of continuous waveforms are used to fit the model output to the actual BP values obtained from a simultaneously taken invasive or intermittent cuff BP measurement, thus determining the subject-specific constants in the model for consequent BP estimation. Although some mechanics-based approaches62,70 are in principle calibration-free, they still require a mapping process to calibrate the local measurement results to the reference BP values obtained from gold standard measurements. Some ML-based algorithms116,121 have demonstrated their capability of performing calibration-free BP estimation, however, their adaptability to untrained subject types and long-term stability are yet to be explored.
Conclusion and future directions
Wearable BP measurement devices have attracted increasing attention from both academia and industry. Several companies (LiveMetric, Healthstats, Sotera, Aktiia, Biobeat, Samsung, etc.) have already commercialized wearable BP solutions and obtained regulatory approval. Yet the realization of accurate and long-term reliable wearable and cuffless BP monitoring systems requires multidisciplinary cooperation. This paper systematically reviewed the devices, signals, algorithms, and other technical issues related to wearable and cuffless BP measurement. Existing sensing technologies cover electrical, optical, and mechanical modalities. Modeling techniques include PTT-based models, ML-based algorithms, and mechanics-based methods. Many challenges and opportunities remain, and future studies may include the following areas:
-
It is noted that many cuffless methods with good performances were validated on small size datasets with only healthy subjects or subjects with a relatively narrow age distribution. To ensure generalizability and domain adaptation of the methods, follow-up clinical studies including a broader range of age groups, CV conditions and BP variation patterns are critical. This trend has also been reflected in the recently established IEEE Standard for Wearable Cuffless Blood Pressure Measuring Devices149, which sets the requirements of subject selection and BP changes to ensure the devices are adequately exposed to both inter- and intra-individual variations.
-
Wearable and cuffless BP monitoring systems should be evaluated under well-established industrial standards to ensure the reliability of the reported measurement performance. The evaluation should include sufficient population size and intra- and inter-individual BP diversity, with a side-by-side comparison of the BP errors between the new and baseline models. In particular, the comparison to generally-accepted baseline models plays a vital role in ML/DL model evaluation because the baseline provides (1) a reasonable performance threshold which the new model should significantly surpass, and (2) reference information of the experimental data for comparison with other studies.
-
Snapshot and beat-to-beat BP monitoring evolved to become mature technologies. Continuous BP monitoring, i.e., TAG tracking, is likely to be the next wave of focused research in the field. DL algorithms will be a powerful tool for achieving TAG monitoring.
-
A comprehensive understanding of both hardware and software is essential for researching wearable and cuffless BP monitoring. Although BP monitoring can be divided to separate tasks, as illustrated in Fig. 1, an end-to-end design that holistically considers the performance characteristics of each technology component should greatly enhance the efficiency and efficacy of the system.
-
Noise is a recurring challenge in BP estimation. Contemporary denoising techniques are effective in the short term. However, maintaining long-term accuracy despite noise is a problem that still requires more feasible solutions.
-
Calibration remains necessary to determine the proper modeling parameters but is inconvenient for users. Current calibration methods ensure accuracy but require user-initiate actions, such as measurements using cuff-based oscillometers. Further development of calibration-free BP monitoring methods is desirable.
-
The fusion of analytical and ML models is promising. Analytic models provide a physiological base for signal selection and processing, whereas ML methods are more effective in data correlation and prediction. Combining the two approaches can not only enhance the BP estimation accuracy but also provide guidelines on the front-end sensor selection and design.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
No new data were generated in this study.
References
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990-2019 update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
Timmis, A. et al. European Society of Cardiology: cardiovascular disease statistics 2019. Eur. Heart J. 41, 12–85 (2020).
Fung, Y.-C. Biomechanics: Circulation (Springer Science & Business Media, 2013).
Lin, J. et al. Wearable sensors and devices for real-time cardiovascular disease monitoring. Cell Rep. Phys. Sci. 2, 100541 (2021).
Inan, O. T. et al. Ballistocardiography and seismocardiography: a review of recent advances. IEEE J. Biomed. Health Inform. 19, 1414–1427 (2015).
Peng, R. C. et al. Cuffless and continuous blood pressure estimation from the heart sound signals. Sensors 15, 23653–23666 (2015).
Liu, S. H., Cheng, D. C. & Su, C. H. A cuffless blood pressure measurement based on the impedance plethysmography technique. Sensors 17, https://doi.org/10.3390/s17051176 (2017).
Ibrahim, B. & Jafari, R. Cuffless blood pressure monitoring from an array of wrist bio-impedance sensors using subject-specific regression models: proof of concept. IEEE Trans. Biomed. Circuits Syst. 13, 1723–1735 (2019).
Chen, S. et al. Flexible wearable sensors for cardiovascular health monitoring. Adv. Healthc. Mater. 10, 2100116 (2021).
Elgendi, M. et al. The use of photoplethysmography for assessing hypertension. npj Digit. Med. 2, https://doi.org/10.1038/s41746-019-0136-7 (2019).
Maqsood, S. et al. A survey: from shallow to deep machine learning approaches for blood pressure estimation using biosensors. Expert Syst. Appl. 197, 24 (2022).
Barvik, D., Cerny, M., Penhaker, M. & Noury, N. Noninvasive continuous blood pressure estimation from pulse transit time: a review of the calibration models. IEEE Rev. Biomed. Eng. 15, 138–151 (2022).
Wang, G., Atef, M. & Lian, Y. Towards a continuous non-invasive cuffless blood pressure monitoring system using PPG: systems and circuits review. IEEE Circuits Syst. Mag. 18, 6–26 (2018).
Mukkamala, R., Stergiou, G. S. & Avolio, A. P. Cuffless blood pressure measurement. Annu. Rev. Biomed. Eng. 24, 203–230 (2022).
Pandit, J. A., Lores, E. & Batlle, D. Cuffless blood pressure monitoring promises and challenges. Clin. J. Am. Soc. Nephrol. 15, 1531–1538 (2020).
Mukkamala, R. et al. Evaluation of the accuracy of cuffless blood pressure measurement devices: challenges and proposals. Hypertension 78, 1161–1167 (2021).
Ding, X. et al. A flexible tonoarteriography-based body sensor network for cuffless measurement of arterial blood pressure. In 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN) 1–4 (2015).
Li, L. et al. Flexible pressure sensors for biomedical applications: from ex vivo to in vivo. Adv. Mater. Interfaces 7, 2000743 (2020).
Mishra, R. B., El‐Atab, N., Hussain, A. M. & Hussain, M. M. Recent progress on flexible capacitive pressure sensors: from design and materials to applications. Adv. Mater. Technol. 6, 2001023 (2021).
He, F. et al. Recent progress in flexible microstructural pressure sensors toward human-machine interaction and healthcare applications. Small Methods 5, e2001041 (2021).
Lin, Q. et al. Highly sensitive flexible iontronic pressure sensor for fingertip pulse monitoring. Adv. Health. Mater. 9, 2001023 (2020).
Luo, N. et al. Hollow-structured graphene–silicone-composite-based piezoresistive sensors: decoupled property tuning and bending reliability. Adv. Mater. 29, 1702675 (2017).
Nichols, W. W. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am. J. Hypertens. 18, 3S–10S (2005).
Luo, Y. et al. Flexible capacitive pressure sensor enhanced by tilted micropillar arrays. ACS Appl. Mater. Interfaces 11, 17796–17803 (2019).
Luo, N. et al. Flexible piezoresistive sensor patch enabling ultralow power cuffless blood pressure measurement. Adv. Funct. Mater. 26, 1178–1187 (2016).
Park, D. Y. et al. Self-powered real-time arterial pulse monitoring using ultrathin epidermal piezoelectric sensors. Adv. Mater. 29, 1702308 (2017).
Meng, K. et al. Flexible weaving constructed self-powered pressure sensor enabling continuous diagnosis of cardiovascular disease and measurement of cuffless blood pressure. Adv. Funct. Mater. 29, 1806388 (2019).
Luo, N. et al. Textile-enabled highly reproducible flexible pressure sensors for cardiovascular monitoring. Adv. Mater. Technol. 3, 1700222 (2018).
Fan, X. et al. Alignment-free liquid-capsule pressure sensor for cardiovascular monitoring. Adv. Funct. Mater. 28, 1805045 (2018).
Hua, Q. et al. Skin-inspired highly stretchable and conformable matrix networks for multifunctional sensing. Nat. Commun. 9, 244 (2018).
Cai, Y.-W. et al. A flexible ultra-sensitive triboelectric tactile sensor of wrinkled PDMS/MXene composite films for E-skin. Nano Energy 81, 105663 (2021).
Bai, N. et al. Graded intrafillable architecture-based iontronic pressure sensor with ultra-broad-range high sensitivity. Nat. Commun. 11, 209 (2020).
Nie, B., Li, R., Cao, J., Brandt, J. D. & Pan, T. Flexible transparent iontronic film for interfacial capacitive pressure sensing. Adv. Mater. 27, 6055–6062 (2015).
Li, R. et al. Research progress of flexible capacitive pressure sensor for sensitivity enhancement approaches. Sens. Actuators A: Phys. 321, 112425 (2021).
Huang, Y., Fan, X., Chen, S. C. & Zhao, N. Emerging technologies of flexible pressure sensors: materials, modeling, devices, and manufacturing. Adv. Funct. Mater. 29, 1808509 (2019).
Lei, H., Chen, Y., Gao, Z., Wen, Z. & Sun, X. Advances in self-powered triboelectric pressure sensors. J. Mater. Chem. A 9, 20100–20130 (2021).
Prasad, A. S., Jayaram, M. N. & Swamy, N. K. Fabrication of GNR electrode for ECG signal acquisition. IEEE Sens. Lett. 5, 4 (2021).
Tan, Y. et al. Dual cross-linked ion-based temperature-responsive conductive hydrogels with multiple sensors and steady electrocardiogram monitoring. Chem. Mat. 32, 7670–7678 (2020).
Xu, S. et al. PEDOT: PSS hydrogel based flexible electrodes for wearable ECG monitoring. In 2020 IEEE SENSORS 1–4 (2020).
Takano, A., Ishigami, H. & Ueno, A. Non-contact measurements of electrocardiogram and cough-associated electromyogram from the neck using in-pillow common cloth electrodes: a proof-of-concept study. Sensors 21, 15 (2021).
Reyes, B. A. et al. Novel electrodes for underwater ECG monitoring. IEEE Trans. Biomed. Eng. 61, 1863–1876 (2014).
Ramasamy, S. & Balan, A. Wearable sensors for ECG measurement: a review. Sens. Rev. 38, 412–419 (2018).
Gandhi, B. & Raghava, N. S. Fabrication techniques for carbon nanotubes based ECG electrodes: a review. IETE J. Res. 1–20, https://doi.org/10.1080/03772063.2020.1768909 (2020).
Lee, K. et al. Neckband-based continuous blood pressure monitoring device with offset-tolerant ROIC. IEEE Access 10, 17300–17309 (2022).
Lee, K. & Yoo, H. J. Simultaneous electrical bio-impedance plethysmography at different body parts: continuous and non-invasive monitoring of pulse wave velocity. IEEE Trans. Biomed. Circ. Syst. 15, 1027–1038 (2021).
Davis, C. L. et al. Direct coextensive plethysmography for non-invasive measurement of systemic pressures and volumes. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vol. 1–7, 2318–2321 (2004).
Santic, A., Stritof, T. & Bilas, V. Plethysmography measurements using short current pulses with low-duty cycle. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vol. 20 Biomedical Engineering Towards the Year 2000 and Beyond 1889–1892 (1998).
Wang, T. W., Chen, W. X., Chu, H. W. & Lin, S. F. Single-channel bioimpedance measurement for wearable continuous blood pressure monitoring. IEEE Trans. Instrum. Meas. 70, https://doi.org/10.1109/TIM.2020.3035578 (2021).
Rachim, V. P., Huynh, T. H. & Chung, W. Wrist photo-plethysmography and bio-impedance sensor for cuff-less blood pressure monitoring. In 2018 IEEE Sensors 1–4 (2018).
Shankar, R. & Webster, J. G. Noninvasive measurement of compliance of human leg arteries. IEEE Trans. Biomed. Eng. 38, 62–67 (1991).
Yang, B., Dong, Y. G., Hou, Z. J. & Xue, X. H. Simultaneously capturing electrocardiography and impedance plethysmogram signals from human feet by capacitive coupled electrode system. IEEE Sens. J. 17, 5654–5662 (2017).
Zheng, Y., Poon, C. C. Y., Yan, B. P. & Lau, J. Y. W. Pulse arrival time based cuff-less and 24-h wearable blood pressure monitoring and its diagnostic value in hypertension. J. Med. Syst. 40, 195 (2016).
Liu, J. et al. Multi-wavelength photoplethysmography method for skin arterial pulse extraction. Biomed. Opt. Express 7, 4313–4326 (2016).
Holz, C. & Wang, E. J. Glabella: continuously sensing blood pressure behavior using an unobtrusive wearable device. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 1, https://doi.org/10.1145/3132024 (2017).
Tamura, T. Current progress of photoplethysmography and SPO2 for health monitoring. Biomed. Eng. Lett. 9, 21–36 (2019).
Lee, H. et al. Toward all-day wearable health monitoring: an ultralow-power, reflective organic pulse oximetry sensing patch. Sci. Adv. 4, eaas9530 (2018).
Polat, E. et al. Flexible graphene photodetectors for wearable fitness monitoring. Sci. Adv. 5, eaaw7846 (2019).
Han, D. et al. Pulse oximetry using organic optoelectronics under ambient light. Adv. Mater. Technol. 5, 1901122 (2020).
Xu, H. et al. Flexible organic/inorganic hybrid near-infrared photoplethysmogram sensor for cardiovascular monitoring. Adv. Mater. 29, 1700975 (2017).
Yokota, T. et al. A conformable imager for biometric authentication and vital sign measurement. Nat. Electron. 3, 113–121 (2020).
Zhou, Q., Lam, K. H., Zheng, H., Qiu, W. & Shung, K. K. Piezoelectric single crystal ultrasonic transducers for biomedical applications. Prog. Mater. Sci. 66, 87–111 (2014).
Zakrzewski, A. M. & Anthony, B. W. Noninvasive blood pressure estimation using ultrasound and simple finite element models. IEEE Trans. Biomed. Eng. 65, 2011–2022 (2018).
Lyon, M. L. & Verma, N. Ultrasound guided volume assessment using inferior vena cava diameter. Open Emerg. Med. J. 3, 22–24 (2010).
Joseph, J., Nabeel, P., Shah, M. I. & Sivaprakasam, M. Arterial compliance probe for calibration free pulse pressure measurement. In 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA) 1–6 (IEEE) (2016).
Rotenberg, M. Y. & Tian, B. Bioelectronic devices: long-lived recordings. Nat. Biomed. Eng. 1, 1–2 (2017).
Han, M. et al. Catheter-integrated soft multilayer electronic arrays for multiplexed sensing and actuation during cardiac surgery. Nat. Biomed. Eng. 4, 997–1009 (2020).
Kwak, J. W. et al. Wireless sensors for continuous, multimodal measurements at the skin interface with lower limb prostheses. Sci. Transl. Med. 12, eabc4327 (2020).
Wang, C. H. et al. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science 377, 517–523 (2022).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018).
Wang, C. et al. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nat. Biomed. Eng. 5, 749–758 (2021).
Zhou, Z. et al. Single-layered ultra-soft washable smart textiles for all-around ballistocardiograph, respiration, and posture monitoring during sleep. Biosens. Bioelectron. 155, 112064 (2020).
Dastjerdi, A. E., Kachuee, M. & Shabany, M. Non-invasive blood pressure estimation using phonocardiogram. In IEEE International Symposium on Circuits and Systems (ISCAS) 80–83 (IEEE, 2017).
Satija, U., Ramkumar, B. & Manikandan, M. S. Automated ECG noise detection and classification system for unsupervised healthcare monitoring. IEEE J. Biomed. Health Inform. 22, 722–732 (2018).
Samartkit, P., Pullteap, S. & Bernal, O. A non-invasive heart rate and blood pressure monitoring system using piezoelectric and photoplethysmographic sensors. Measurement 196, 111211 (2022).
Hu, H. et al. Stretchable ultrasonic transducer arrays for three-dimensional imaging on complex surfaces. Sci. Adv. 4, eaar3979 (2018).
Fati, S. M., Muneer, A., Akbar, N. A. & Taib, S. M. A continuous cuffless blood pressure estimation using tree-based pipeline optimization tool. Symmetry 13, 22 (2021).
Huang, Y. P., Song, Y. T., Gou, L. & Zou, Y. N. A novel wearable flexible dry electrode based on cowhide for ECG measurement. Biosensors 11, 17 (2021).
Satti, A. T., Park, J., Park, J., Kim, H. & Cho, S. Fabrication of parylene-coated microneedle array electrode for wearable ECG device. Sensors 20, 15 (2020).
Pashaei, V. et al. Flexible body-conformal ultrasound patches for image-guided neuromodulation. IEEE Trans. Biomed. circuits Syst. 14, 305–318 (2019).
Kim, C.-S., Carek, A. M., Mukkamala, R., Inan, O. T. & Hahn, J.-O. Ballistocardiogram as proximal timing reference for pulse transit time measurement: potential for cuffless blood pressure monitoring. IEEE Trans. Biomed. Eng. 62, 2657–2664 (2015).
Poh, M.-Z., Swenson, N. C. & Picard, R. W. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans. Inf. Technol. Biomed. 14, 786–794 (2010).
Ibrahim, B. & Jafari, R. Continuous blood pressure monitoring using wrist-worn bio-impedance sensors with wet electrodes. In 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS) (IEEE, 2018).
Mukkamala, R. et al. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans. Biomed. Eng. 62, 1879–1901 (2015).
Ding, X.-R. et al. An attempt to define the pulse transit time. In International Conference on Biomedical and Health Informatics 219–221 (Springer, 2019).
Bramwell, J. C. & Hill, A. V. The velocity of pulse wave in man. Proc. R. Soc. Lond. Ser. B, Containing Pap. a Biol. Character 93, 298–306 (1922).
Hughes, D. Measurements of Young’s modulus of elasticity of the canine aorta with ultrasound. Ultrason. Imaging 1, 356–367 (1979).
Obrist, P. A., Light, K. C., McCubbin, J. A., Hutcheson, J. S. & Hoffer, J. L. Pulse transit-time - relationship to blood-pressure. Behav. Res. Methods Instrum. 10, 623–626 (1978).
Chen, W., Kobayashi, T., Ichikawa, S., Takeuchi, Y. & Togawa, T. Continuous estimation of systolic blood pressure using the pulse arrival time and intermittent calibration. Med. Biol. Eng. Comput. 38, 569–574 (2000).
Poon, C. C. Y. & Zhang, Y. T. Cuff-less and noninvasive measurements of arterial blood pressure by pulse transit time. In 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference (IEEE, 2005).
Wong, M. Y.-M., Poon, C. C.-Y. & Zhang, Y.-T. An evaluation of the cuffless blood pressure estimation based on pulse transit time technique: a half year study on normotensive subjects. Cardiovasc. Eng. 9, 32–38 (2009).
Gesche, H., Grosskurth, D., Küchler, G. & Patzak, A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur. J. Appl. Physiol. 112, 309–315 (2012).
Ding, X.-R., Zhang, Y.-T., Liu, J., Dai, W.-X. & Tsang, H. K. Continuous cuffless blood pressure estimation using pulse transit time and photoplethysmogram intensity ratio. IEEE Trans. Biomed. Eng. 63, 964–972 (2016).
Liu, J. et al. Multi-wavelength photoplethysmography enabling continuous blood pressure measurement with compact wearable electronics. IEEE Trans. Biomed. Eng. 66, 1514–1525 (2018).
Huynh, T., Jafari, R. & Chung, W.-Y. An accurate bioimpedance measurement system for blood pressure monitoring. Sensors 18, 2095 (2018).
Huynh, T. H., Jafari, R. & Chung, W.-Y. Noninvasive cuffless blood pressure estimation using pulse transit time and impedance plethysmography. IEEE Trans. Biomed. Eng. 66, 967–976 (2019).
Rachim, V. P. & Chung, W.-Y. Multimodal wrist biosensor for wearable cuff-less blood pressure monitoring system. Sci. Rep. 9, https://doi.org/10.1038/s41598-019-44348-3 (2019).
He, D. D., Winokur, E. S. & Sodini, C. G. An ear-worn vital signs monitor. IEEE Trans. Biomed. Eng. 62, 2547–2552 (2015).
Mieloszyk, R. et al. A comparison of wearable tonometry, photoplethysmography, and electrocardiography for cuffless measurement of blood pressure in an ambulatory setting. IEEE J. Biomed. Health Inform. 26, 2864–2875 (2022).
Mukkamala, R. et al. The microsoft research aurora project: important findings on cuffless blood pressure measurement. Hypertension 0, https://doi.org/10.1161/HYPERTENSIONAHA.122.20410.
Natarajan, K. et al. Photoplethysmography fast upstroke time intervals can be useful features for cuff-less measurement of blood pressure changes in humans. IEEE Trans. Biomed. Eng. 69, 53–62 (2022).
Figini, V. et al. Improving cuff-less continuous blood pressure estimation with linear regression analysis. Electronics 11, 1442 (2022).
Mamun, M. Cuff-less blood pressure measurement based on hybrid feature selection algorithm and multi-penalty regularized regression technique. Biomed. Phys. Eng. Express 7, 16 (2021).
Liu, Q., Zheng, Y. L., Zhang, Y. T. & Poon, C. C. Y. Beats-to-beats estimation of blood pressure during supine cycling exercise using a probabilistic nonparametric method. IEEE Access 9, 115655–115663 (2021).
Yang, S. et al. Blood pressure estimation with complexity features from electrocardiogram and photoplethysmogram signals. Opt. Quantum Electron. 52, https://doi.org/10.1007/s11082-020-2260-7 (2020).
Jean Effil, N. & Rajeswari, R. Wavelet scattering transform and long short-term memory network-based noninvasive blood pressure estimation from photoplethysmograph signals. Signal, Image Video Process. 16, 1–9 (2021).
Saeed, M. et al. Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC-II): a public-access intensive care unit database. Crit. Care Med. 39, 952 (2011).
Zhang, Q. X., Zeng, X., Hu, W. C. & Zhou, D. A. A machine learning-empowered system for long-term motion-tolerant wearable monitoring of blood pressure and heart rate with ear-ECG/PPG. IEEE Access 5, 10547–10561 (2017).
Chang, C.-C. & Lin, C.-J. LIBSVM: a library for support vector machines. ACM Trans. Intell. Syst. Technol. 2, https://doi.org/10.1145/1961189.1961199 (2011).
Chen, Y., Cheng, J. & Ji, W. Q. Continuous blood pressure measurement based on photoplethysmography. In 14th IEEE International Conference on Electronic Measurement and Instruments (ICEMI) 1656–1663 (IEEE, 2019).
Fang, Y. F., Huang, P. W., Chung, M. L. & Wu, B. F. A feature selection method for vision-based blood pressure measurement. In IEEE International Conference on Systems, Man, and Cybernetics (SMC) 2158–2163 (IEEE, IEEE Syst Man & Cybernet Soc, 2018).
Nath, R. K. & Thapliyal, H. PPG based continuous blood pressure monitoring framework for smart home environment. In 6th IEEE Virtual World Forum on Internet of Things (IEEE WF-IoT) (IEEE, Electr Network, 2020).
Gupta, S., Singh, A. & Sharma, A. Dynamic large artery stiffness index for cuffless blood pressure estimation. IEEE Sens. Lett. 6, 4 (2022).
Farki, A., Kazemzadeh, R. B. & Noughabi, E. A. A novel clustering-based algorithm for continuous and noninvasive cuff-less blood pressure estimation. J. Healthc. Eng. 2022, 13 (2022).
Ma, K. S. K., Hao, H., Huang, H. C. & Tang, Y. H. Entropy-facilitated machine learning for blood pressure estimation using electrocardiogram and photoplethysmogram in a wearable device. In 14th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI) (IEEE, 2021).
Baek, S., Jang, J. & Yoon, S. End-to-end blood pressure prediction via fully convolutional networks. IEEE Access 7, 185458–185468 (2019).
Esmaelpoor, J., Sanat, Z. M. & Moradi, M. H. A clinical set-up for noninvasive blood pressure monitoring using two photoplethysmograms and based on convolutional neural networks. Biomed. Tech. 66, 375–385 (2021).
Liu, Z. et al. Cuff-less blood pressure measurement based on deep convolutional neural network. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 3775–3778 (2019).
Qiu, Y. et al. Cuffless blood pressure estimation based on composite neural network and graphics information. Biomed. Signal Process. Control 70, https://doi.org/10.1016/j.bspc.2021.103001 (2021).
Fan, X., Wang, H., Xu, F., Zhao, Y. & Tsui, K. L. Homecare-oriented intelligent long-term monitoring of blood pressure using electrocardiogram signals. IEEE Trans. Ind. Inform. 16, 7150–7158 (2020).
Fan, X., Wang, H., Zhao, Y., Li, Y. & Tsui, K. L. An adaptive weight learning-based multitask deep network for continuous blood pressure estimation using electrocardiogram signals. Sensors 21, 1–18 (2021).
El-Hajj, C. & Kyriacou, P. A. Deep learning models for cuffless blood pressure monitoring from PPG signals using attention mechanism. Biomed. Signal Process. Control 65, https://doi.org/10.1016/j.bspc.2020.102301 (2021).
Radha, M. et al. Estimating blood pressure trends and the nocturnal dip from photoplethysmography. Physiol. Meas. 40, https://doi.org/10.1088/1361-6579/ab030e (2019).
Li, Y. H., Harfiya, L. N., Purwandari, K. & Lin, Y. D. Real-time cuffless continuous blood pressure estimation using deep learning model. Sensors 20, 1–19 (2020).
Paviglianiti, A., Randazzo, V., Villata, S., Cirrincione, G. & Pasero, E. A comparison of deep learning techniques for arterial blood pressure prediction. Cogn. Comput. 14, 1689–1710 (2021).
Senturk, U., Yucedag, I. & Polat, K. Repetitive neural network (RNN) based blood pressure estimation using PPG and ECG signals. In ISMSIT 2018 - 2nd International Symposium on Multidisciplinary Studies and Innovative Technologies, Proceedings (2018).
Baker, S., Xiang, W. & Atkinson, I. A hybrid neural network for continuous and non-invasive estimation of blood pressure from raw electrocardiogram and photoplethysmogram waveforms. Comput. Methods Programs Biomed. 207, https://doi.org/10.1016/j.cmpb.2021.106191 (2021).
El-Hajj, C. & Kyriacou, P. A. Cuffless blood pressure estimation from PPG signals and its derivatives using deep learning models. Biomed. Signal Process. Control 70, https://doi.org/10.1016/j.bspc.2021.102984 (2021).
Eom, H. et al. End-to-end deep learning architecture for continuous blood pressure estimation using attention mechanism. Sensors 20, 2338 (2020).
Panwar, M., Gautam, A., Biswas, D. & Acharyya, A. PP-Net: a deep learning framework for PPG-based blood pressure and heart rate estimation. IEEE Sens. J. 20, 10000–10011 (2020).
Rong, M. & Li, K. A multi-type features fusion neural network for blood pressure prediction based on photoplethysmography. Biomed. Signal Process. Control 68, https://doi.org/10.1016/j.bspc.2021.102772 (2021).
Leitner, J., Chiang, P. H. & Dey, S. Personalized blood pressure estimation using photoplethysmography: a transfer learning approach. IEEE J. Biomed. Health Inf. 26, 218–228 (2022).
Yang, S., Zhang, Y., Cho, S. Y., Correia, R. & Morgan, S. P. Non-invasive cuff-less blood pressure estimation using a hybrid deep learning model. Opt. Quantum Electron. 53, https://doi.org/10.1007/s11082-020-02667-0 (2021).
Schrumpf, F., Frenzel, P., Aust, C., Osterhoff, G. & Fuchs, M. Assessment of non-invasive blood pressure prediction from ppg and rppg signals using deep learning. Sensors 21, https://doi.org/10.3390/s21186022 (2021).
Wang, W. et al. Photoplethysmography-based blood pressure estimation using deep learning. In Conference Record - Asilomar Conference on Signals, Systems and Computers 945–949 (2020).
Kissas, G. et al. Machine learning in cardiovascular flows modeling: predicting arterial blood pressure from non-invasive 4D flow MRI data using physics-informed neural networks. Comput. Methods Appl. Mech. Eng. 358, https://doi.org/10.1016/j.cma.2019.112623 (2020).
Esper, S. A. & Pinsky, M. R. Arterial waveform analysis. Best. Pract. Res. Clin. Anaesthesiol. 28, 363–380 (2014).
Athaya, T. & Choi, S. An estimation method of continuous non-invasive arterial blood pressure waveform using photoplethysmography: A u-net architecture-based approach. Sensors 21, 1–18 (2021).
Cheng, J. et al. Prediction of arterial blood pressure waveforms from photoplethysmogram signals via fully convolutional neural networks. Comput. Biol. Med. 138, https://doi.org/10.1016/j.compbiomed.2021.104877 (2021).
Sadrawi, M. et al. Genetic deep convolutional autoencoder applied for generative continuous arterial blood pressure via photoplethysmography. Sensors 20, 1–18 (2020).
Qin, K., Huang, W. & Zhang, T. Deep generative model with domain adversarial training for predicting arterial blood pressure waveform from photoplethysmogram signal. Biomed. Signal Process. Control 70, https://doi.org/10.1016/j.bspc.2021.102972 (2021).
Hill, B. L. et al. Imputation of the continuous arterial line blood pressure waveform from non-invasive measurements using deep learning. Sci. Rep. 11, 15755 (2021).
Aguirre, N., Grall-Maës, E., Cymberknop, L. J. & Armentano, R. L. Blood pressure morphology assessment from photoplethysmogram and demographic information using deep learning with attention mechanism. Sensors 21, 1–19 (2021).
Harfiya, L. N., Chang, C. C. & Li, Y. H. Continuous blood pressure estimation using exclusively photopletysmography by lstm-based signal-to-signal translation. Sensors 21, https://doi.org/10.3390/s21092952 (2021).
Sideris, C., Kalantarian, H., Nemati, E. & Sarrafzadeh, M. Building continuous arterial blood pressure prediction models using recurrent networks. in 2016 IEEE International Conference on Smart Computing (SMARTCOMP) 1–5 (2016).
Kim, T. et al. Flexible 1–3 composite ultrasound transducers with Silver-Nanowire-Based stretchable electrodes. IEEE Trans. Ind. Electron. 67, 6955–6962 (2019).
Milne, L. et al. Central aortic blood pressure from ultrasound wall-tracking of the carotid artery in children: comparison with invasive measurements and radial tonometry. Hypertension 65, 1141–1146 (2015).
Arndt, J. O., Klauske, J. & Mersch, F. The diameter of the intact carotid artery in man and its change with pulse pressure. Pflugers Arch. Gesamte Physiol. Menschen Tiere 301, 230–240 (1968).
IEEE Standard Association. IEEE standard for wearable cuffless blood pressure measuring devices. IEEE Std 1708-2014, 1–38 (2014).
Yang, J. et al. Facile fabrication of robust and reusable PDMS supported graphene dry electrodes for wearable electrocardiogram monitoring. Adv. Mater. Technol. 6, 10 (2021).
Yeu, R., Ra, N., Lee, S. A. & Nam, Y. Evaluation of pencil lead based electrodes for electrocardiogram monitoring in hot spring. CMC-Comput. Mat. Contin. 66, 1411–1425 (2021).
Ren, K. et al. Contact ECG recording using copper and E-textile based flexible dry electrodes. Prog. Electromagn. Res. M 101, 47–58 (2021).
Pradhan, U. U., Reddy, N., Chandrashekar, K. & Mohan, C. B. Titanium dioxide based bioelectric sensor for the acquisition of electrocardiogram signals. Microchem. J. 160, 7 (2021).
Li, X. S. et al. Roll-to-roll graphene films for non-disposable electrocardiogram electrodes. J. Phys. D.-Appl. Phys. 54, 7 (2021).
Huang, C. Y. & Chiu, C. W. Facile fabrication of a stretchable and flexible nanofiber carbon film-sensing electrode by electrospinning and its application in smart clothing for ECG and EMG monitoring. ACS Appl. Electron. Mater. 3, 676–686 (2021).
Zhang, K. et al. Skin conformal and antibacterial PPy-leather electrode for ECG monitoring. Adv. Electron. Mater. 6, 8 (2020).
Tasneem, N. T., Pullano, S. A., Critello, C. D., Fiorillo, A. S. & Mahbub, I. A low-power on-chip ECG monitoring system based on MWCNT/PDMS dry electrodes. IEEE Sens. J. 20, 12799–12806 (2020).
Tan, R. L. S., Tey, J. N., Kerk, W. T. & Lok, B. K. Roll-to-roll manufacturing of printed biosensor electrodes for ECG monitoring. in 22nd IEEE Electronics Packaging Technology Conference (EPTC) 213-215 (IEEE, Electr Network, 2020).
Suzuki, S. & Oguri, K. Cuffless and non-invasive Systolic Blood Pressure estimation for aged class by using a Photoplethysmograph. in 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 1327–1330 (IEEE, 2008).
Suzuki, A. & Ryu, K. Feature selection method for estimating systolic blood pressure using the Taguchi method. IEEE Trans. Ind. Inform. 10, 1077–1085 (2014).
Duan, K. F., Qian, Z. L., Atef, M. & Wang, G. X. A feature exploration methodology for learning based cuffless blood pressure measurement using photoplethysmography. in 38th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC) 6385–6388 (IEEE, 2016).
Gayapersad, N., Rocke, S., Ramsaroop, Z., Singh, A. & Ramlal, C. Beyond blood pressure and heart rate monitoring: towards a device for continuous sensing and automatic feature extraction of cardiovascular data. in 8th International Conference on Computational Intelligence and Communication Networks (CICN) 261–265 (IEEE Computer Soc, 2016).
Meng, L. F., Zhang, Z. Q., Miao, Y., Xie, X. Q. & Pan, H. W. A multi-feature fusion method to estimate blood pressure by PPG. in International Conference for Smarth Health (ICSH) 121–131 (Springer International Publishing Ag, 2017).
Lin, C., Zhou, Y., Wang, H. & Wang, Y. Pulse waveform as an indicator of baseline offset in pulse transit time based blood pressure estimation. in NIH-IEEE Strategic Conference on Healthcare Innovations and Point-of-Care Technologies (HI-POCT) 26–31 (IEEE, 2017).
Lin, W. H. et al. New photoplethysmogram indicators for improving cuffless and continuous blood pressure estimation accuracy. Physiol. Meas. 39, 13 (2018).
Zadi, A. S., Alex, R., Zhang, R., Watenpaugh, D. E. & Behbehani, K. Arterial blood pressure feature estimation using photoplethysmography. Comput. Biol. Med. 102, 104–111 (2018).
Attarpour, A., Mahnam, A., Aminitabar, A. & Samani, H. Cuff-less continuous measurement of blood pressure using wrist and fingertip photo-plethysmograms: Evaluation and feature analysis. Biomed. Signal Process. Control 49, 212–220 (2019).
Hassani, A. & Foruzan, A. H. Improved PPG-based estimation of the blood pressure using latent space features. Signal Image Video Process 13, 1141–1147 (2019).
Rundo, F., Ortis, A., Battiato, S. & Conoci, S. Advanced multi-neural system for cuff-less blood pressure estimation through nonlinear HC-features. in 16th International Joint Conference on E-Business and Telecommunications (ICETE) 321–325 (Scitepress, 2019).
Chowdhury, M. H. et al. Estimating blood pressure from the photoplethysmogram signal and demographic features using machine learning techniques. Sensors 20, 24 (2020).
Hasanzadeh, N., Ahmadi, M. M. & Mohammadzade, H. Blood pressure estimation using photoplethysmogram signal and its morphological features. IEEE Sens. J. 20, 4300–4310 (2020).
Lin, W. H., Li, X. X., Li, Y. H., Li, G. L. & Chen, F. Investigating the physiological mechanisms of the photoplethysmogram features for blood pressure estimation. Physiol. Meas. 41, 13 (2020).
Singla, M., Azeemuddin, S. & Sistla, P. Learning-based model for central blood pressure estimation using feature extracted from ECG and PPG signals. in 42nd Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC) 855–858 (IEEE, 2020).
Thambiraj, G., Gandhi, U., Mangalanathan, U., Jose, V. J. M. & Anand, M. Investigation on the effect of Womersley number, ECG and PPG features for cuff less blood pressure estimation using machine learning. Biomed. Signal Process. Control 60, 11 (2020).
Esmaelpoor, J., Moradi, M. H. & Kadkhodamohammadi, A. Cuffless blood pressure estimation methods: physiological model parameters versus machine-learned features. Physiol. Meas. 42, 19 (2021).
Maqsood, S., Xu, S., Springer, M. & Mohawesh, R. A benchmark study of machine learning for analysis of signal feature extraction techniques for blood pressure estimation using photoplethysmography (PPG). IEEE Access 9, 138817–138833 (2021).
Ding, X. R. et al. Coherence analysis of invasive blood pressure and its noninvasive indicators for improvement of cuffless measurement accuracy. In 39th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC) 2255–2258 (IEEE, 2017).
Osawa, Y., Hata, S., Hori, M. & Dohi, T. Comparison of features by simultaneous measurement of blood pressure pulse wave and electrocardiogram. In 42nd Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC) 4664–4667 (IEEE, 2020).
Ramakrishna, P., Nabeel, P. M., Raj Kiran, V., Joseph, J. & Sivaprakasam, M. Cuffless blood pressure estimation using features extracted from carotid dual-diameter waveforms. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2719–2722 (2020).
Peng, C., Chen, M., Sim, H. K., Zhu, Y. & Jiang, X. Noninvasive and nonocclusive blood pressure monitoring via a flexible piezo-composite ultrasonic sensor. IEEE Sens. J. 21, 2642–2650 (2021).
Jana, B., Oswal, K., Mitra, S., Saha, G. & Banerjee, S. Windkessel model-based cuffless blood pressure estimation using continuous wave doppler ultrasound system. IEEE Sens. J. 20, 9989–9999 (2020).
Liu, Z.-D. et al. Cuffless blood pressure estimation using pressure pulse wave signals. Sensors 18, 4227 (2018).
Huang, K. H., Tan, F., Wang, T. D. & Yang, Y. J. A highly sensitive pressure-sensing array for blood pressure estimation assisted by machine-learning techniques. Sensors 19, 15 (2019).
Singla, M., Sistla, P. & Azeemuddin, S. Cuff-less blood pressure measurement using supplementary ECG and PPG features extracted through wavelet transformation. In 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 4628–4631 (IEEE, 2019).
Lin, W. H. et al. Towards accurate estimation of cuffless and continuous blood pressure using multi-order derivative and multivariate photoplethysmogram features. Biomed. Signal Process. Control 63, 12 (2021).
Song, K., Park, T. J. & Chang, J. H. Novel data augmentation employing multivariate gaussian distribution for neural network-based blood pressure estimation. Appl. Sci. 11, https://doi.org/10.3390/app11093923 (2021).
Zhang, Y. D., Zhang, X. W., Cui, P. F., Li, S. & Tang, J. T. Key feature selection and model analysis for blood pressure estimation from electrocardiogram, ballistocardiogram and photoplethysmogram. IEEE Access 9, 54350–54359 (2021).
Wang, Y.-J., Chen, C.-H., Sue, C.-Y., Lu, W.-H. & Chiou, Y.-H. Estimation of blood pressure in the radial artery using strain-based pulse wave and photoplethysmography sensors. Micromachines 9, 556 (2018).
Ding, X. R. & Zhang, Y. T. Photoplethysmogram intensity ratio: a potential indicator for improving the accuracy of PTT-based cuffless blood pressure estimation. In 37th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC) 398–401 (IEEE, 2015).
Lin, W. H., Wang, H., Samuel, O. W. & Li, G. L. Using a new PPG indicator to increase the accuracy of PTT-based continuous cuffless blood pressure estimation. in 39th Annual International Conference of the IEEE-Engineering-in-Medicine-and-Biology-Society (EMBC) 738–741 (IEEE, 2017).
Yang, S. M., Sohn, J., Lee, S., Lee, J. & Kim, H. C. Estimation and validation of arterial blood pressure using photoplethysmogram morphology features in conjunction with pulse arrival time in large open databases. IEEE J. Biomed. Health Inform. 25, 1018–1030 (2021).
Jiang, H. Y. et al. A novel indicator for cuff-less blood pressure estimation based on photoplethysmography. In 5th International Conference on Health Information Science (HIS) 119–128 (Springer International Publishing Ag, 2016).
Wang, D. L. et al. Photoplethysmography-based blood pressure estimation combining filter-wrapper collaborated feature selection with LASSO-LSTM model. IEEE Trans. Instrum. Meas. 70, 14 (2021).
Simjanoska, M., Gjoreski, M., Gams, M. & Madevska Bogdanova, A. Non-invasive blood pressure estimation from ECG using machine learning techniques. Sensors 18, 1160 (2018).
Tamura, T., Maeda, Y., Sekine, M. & Yoshida, M. Wearable photoplethysmographic sensors—past and present. Electronics 3, 282–302 (2014).
Acknowledgements
This work was supported in part by InnoHK Project on “Wearable Intelligent Sensor Engineering (WISE) and Bioinstrumentation for the Monitoring of CVD Risk Factors” at Hong Kong Centre for Cerebro-cardiovascular Health Engineering (COCHE) and in part by the Excellent Young Scientists Fund from National Natural Science Foundation of China (62022004).
Author information
Authors and Affiliations
Contributions
N.Z. and L.Z. conceived and designed the study. N.Z., L.Z., C.L., Y.H., G.Z., Y.X., N.J., and Y.-T. Z. wrote and revised the paper. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, L., Liang, C., Huang, Y. et al. Emerging sensing and modeling technologies for wearable and cuffless blood pressure monitoring. npj Digit. Med. 6, 93 (2023). https://doi.org/10.1038/s41746-023-00835-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-023-00835-6
- Springer Nature Limited
This article is cited by
-
Flexible adaptive sensing tonometry for medical-grade multi-parametric hemodynamic monitoring
npj Flexible Electronics (2024)
-
Video-based beat-by-beat blood pressure monitoring via transfer deep-learning
Applied Intelligence (2024)
-
Challenges and prospects of visual contactless physiological monitoring in clinical study
npj Digital Medicine (2023)
-
Current and Developing Technologies for BP Monitoring
Current Cardiology Reports (2023)