Abstract
Neurotrophic tyrosine kinase (NTRK) fusions involving NTRK1, NTRK2, and NTRK3 were found in a broad range of solid tumors as driver gene variants. However, the prevalence of NTRK fusions in Chinese solid tumor patients is rarely reported. Based on the next-generation sequencing data from 10,194 Chinese solid tumor patients, we identified approximately 0.4% (40/10,194) of Chinese solid tumor patients with NTRK fusion. NTRK fusions were most frequently detected in soft tissue sarcoma (3.0%), especially in the fibrosarcoma subtype (12.7%). A total of 29 NTRK fusion patterns were identified, of which 11 were rarely reported. NTRK fusion mostly co-occurred with TP53 (38%), CDKN2A (23%), and ACVR2A (18%) and rarely with NTRK amplification (5.0%) and single nucleotide variants (2.5%). DNA-based NTRK fusion sequencing exhibited a higher detection rate than pan-TRK immunohistochemistry (100% vs. 87.5%). Two patients with NTRK fusions showed clinical responses to larotrectinib, supporting the effective response of NTRK fusion patients to TRK inhibitors.
Similar content being viewed by others
Introduction
Neurotrophic tyrosine kinase (NTRK) genes, involving NTRK1, NTRK2, and NTRK3, encode the three transmembrane proteins, tropomyosin receptor kinase (TRK) A, B, and C of the TRK family, which are the receptors of nerve growth factor (NGF), brain-derived growth factor (BDNF), and neurotrophin 3 (NTF-3), respectively1,2. TRK receptors play important roles in the development and homeostasis of the nervous system. The NTRK1, NTRK2, and NTRK3 genes are located on chromosomes 1q23.1, 9q22.1, and 15q25, respectively3.
NTRK fusions caused by interchromosomal or intrachromosomal rearrangement represent functional genomic alterations in many cancers. The chimeric oncoprotein containing the TRK tyrosine kinase domain is constitutively activated and/or overexpressed, resulting in the activation of downstream pro-oncogenic pathways4. NTRK fusions were found in about 0.3% of solid tumors, and the frequency of fusion was different by cancer types5. NTRK fusions have recently been identified as targets for cancer therapy as well6, culminating in the first global approval by the Food and Drug Administration (FDA) for Larotrectinib in November 2018, followed by approval in more than 45 countries, including China, for the treatment of adult and pediatric patients with metastatic or locally advanced solid tumors harboring an NTRK fusion gene. Notably, due to the broad durable activity across a range of cancer types, this was the first approval of tissue agnostic drug for any solid tumors harboring an NTRK fusion7.
More than 80 partner genes have been reported to fuse with three NTRK genes, generating even more chimeric proteins8. Therefore, the classic assays for gene rearrangement, such as fluorescent in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR), are incompetent for the detection of complex NTRK fusions, such as different fusion partners and breakpoints. Next-generation sequencing (NGS)-based detections, particularly those utilizing RNA as a starting material, are recommended for NTRK fusion detection9,10,11.
Exploration of the incidence of NTRK fusions in a variety of solid tumors is limited12; in particular, data from a large cohort of Chinese patients with solid tumors has not been reported. In this study, we detected and comprehensively characterized the prevalence of NTRK fusions in more than 10,000 Chinese patients with solid tumors and further investigated whether NGS is a reliable method for NTRK fusion detection in clinical practice.
Results
Patient characteristics
The clinical characteristics of 10,194 patients enrolled in this study are summarized in Table 1. The cohort (Supplementary Fig. 1, Table 1) included non-small cell lung cancer (N = 2039, 20.0%), colorectal cancer (N = 1225, 12.0%), hepatocellular carcinoma (N = 1133, 11.1%), gastric cancer (N = 866, 8.5%), esophageal carcinoma (N = 610, 6.0%), soft tissue sarcoma (N = 571, 5.6%), intrahepatic cholangiocarcinoma (N = 555, 5.4%), pancreatic cancer (N = 498, 4.9%), extrahepatic cholangiocarcinoma (N = 351, 3.4%), breast cancer (N = 323, 3.2%), renal cell carcinoma (N = 308, 3.0%), ovarian cancer (N = 261, 2.6%), gallbladder carcinoma (N = 240, 2.4%), small cell lung cancer (N = 220, 2.2%), bone sarcoma (N = 183, 1.8%), head and neck carcinoma (N = 175, 1.7%), cancer of unknown primary (N = 120, 1.2%), endocervical carcinoma (N = 104, 1.0%), and others (N = 412, 4.0%). The median age of patients was 58 years, ranging from ≤1 to 96 years. In the cohort, 60.4% were male and 39.6% were female.
In this cohort, there were 40 patients with NTRK fusion, 13 of which underwent multiple detection methods, including DNA-seq, RNA-seq, and immunohistochemistry (IHC). There was a significant difference in the age distribution between NTRK fusion-positive (N = 40, 0.4%) and NTRK fusion-negative (N = 10,154) patients (median age, 45 vs. 58 years, respectively, P < 0.01).
Among 40 patients with NTRK fusions, 4 cases arose in infants (age ≤ 1 year), 6 in pediatric patients (age range from 1 to 18 years), and 30 in adult patients (age > 18 years). Among 10 NTRK fusion-positive infantile/pediatric patients (age ≤ 18 years), there were 10 soft tissue sarcomas, including 6 infantile fibrosarcomas, 1 cellular plexiform schwannoma, 1 rhabdomyosarcoma, and 2 soft tissue sarcomas of the abdomen (Tables 1 and 2). However, there was no apparent difference in sex and tumor stage distribution between NTRK fusion-positive and fusion-negative patients. Although tumor mutational burden (TMB) value was comparable between these two cohorts (NTRK fusion-positive vs. NTRK fusion-negative: 4.6 [0–180] vs. 4.6 [0–825] muts/Mb, P = 0.19), NTRK fusions were enriched in tumors with high microsatellite instability (MSI-H) (MSI-H vs. microsatellite stability [MSS]: 3.2% [6/186] vs. 0.34% [32/9461]; P < 0.001), including 4 colorectal cancers, 1 gastric cancer, and 1 intrahepatic cholangiocarcinoma (Tables 1 and 2).
NTRK fusions
NTRK fusion was predicted to be a functional TRK fusion protein, including “definite fusion” of in-strand and in-frame states with the NTRK kinase domain and “likely fusion” of out-frame states in a known partner gene or in a hot exon with the NTRK kinase domain.
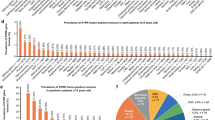
In this cohort, a total of 41 NTRK fusions were detected in 40 patients, including 34 NTRK definite fusions and 7 NTRK likely fusions (Table 2). The incidence of NTRK fusions in tumor types was as follows: 3.0% in soft tissue sarcoma (17/571), 3.1% in thyroid tumor (1/32), 0.9% in small cell lung cancer (2/220), 0.6% in colorectal cancer (7/1225) and head and neck carcinoma (1/175), 0.4% in ovarian cancer (1/261), 0.3% in extrahepatic cholangiocarcinoma (1/351) and breast cancer (1/323), 0.2% in non-small cell lung cancer (5/2039), hepatocellular carcinoma (2/1133), and intrahepatic cholangiocarcinoma (1/555), and 0.1% in gastric cancer (1/866) (Fig. 1a), and detailed histologic diagnosis of these cases were listed in Table 2. There were 20 (48.8%) NTRK1 and 21 (51.2%) NTRK3 fusions in 7 and 12 tumor types, respectively (Fig. 1a). No NTRK2 fusion was detected in this cohort.
a Distribution of NTRK fusion in various tumor types. The frequency of NTRK fusion in each cancer was denoted on the top of the bar. b Distribution of partner genes in NTRK fusions. c Structure of 29 NTRK fusion patterns and the positions of each breakpoint. The middle horizontal line distinguishes the fusions of NTRK1 and NTRK3, the middle vertical line represents the breakpoint of gene fusions, and the orange color boxes represent the kinase domain of the NTRK genes. Asterisks indicate novel fusion patterns of NTRK genes.
The cohort included 55 fibrosarcoma patients, and 7 (12.7%) were NTRK fusion-positive. Given the age of these patients, NTRK fusion occurred significantly more frequently in infantile fibrosarcoma (age ≤ 18 years) (6/12) than in adult fibrosarcoma (age 18 years, 1/43) (P < 0.001).
Overall, 21 unique fusion partners were identified in our NTRK fusion-positive cohort (N = 40, Table 2). Several fusions are well known, including ETV6-NTRK3 (N = 10), LMNA-NTRK1 (N = 7), TPM3-NTRK1 (N = 6), and TPR-NTRK1 (N = 1); 11 were rare fusions (26.8%, 11/41) including 2 rare partners with NTRK1 and 9 rare partners with NTRK3 (Fig. 1b). NTRK fusion patterns and the exon composition of NTRK and their partners were identified in our study. Totally, NTRK3 fusions had the most diverse partners (N = 12), followed by NTRK1 fusions (N = 9). In our cohort, there was no fusion partner shared by NTRK1 and NTRK3 (Fig. 1c). All of the fusions preserved an intact tyrosine kinase domain, which could lead to constitutive activation of TRK receptors. NTRK1 breakpoints were located on a broad range of introns, including introns 3, 4, 7, 8, 9, 10, 11, and 13, and exons 8, 10, and 11. By contrast, NTRK3 breakpoints were limited to intron 13 and intron 14. This suggested that NTRK1 and NTRK 3 had distinctive fusion patterns in our cohort. Furthermore, intrachromosomal rearrangements comprised the most fusions of NTRK1 (90%, 18/20), whereas interchromosomal rearrangements comprised the vast majority of NTRK3 fusions (85.7%, 18/21) (Table 2). Most rare fusion partners and 70% (7/10) of known fusion partners were present only once. Additionally, breakpoints were distributed in different exons of the NTRK genes, especially for NTRK1, suggesting that NTRK fusions were heterogeneous in Chinese solid tumor patients.
Co-occurring genomic alterations in NTRK fusion-positive patients
One of the advantages of NGS technology is the simultaneous discovery of various genomic variants. The co-occurrence of multi-genomic alterations was identified in patients with NTRK fusions (Fig. 2).
The X-axis represents each patient and the Y-axis represents each mutated gene. The bar graph above shows the information of tumor mutational burden (TMB), age, MSI, and gender of each patient, and the bar graph on the right shows the mutation number of each sample. Green represents substitution/Indel mutations, red represents gene amplification mutations, blue represents gene homozygous deletion mutations, yellow represents fusion/rearrangement mutations, and purple represents truncation mutations.
Among 40 cases with NTRK fusions, 30% (12 of 40) harbored alterations that activated the downstream PI3K signaling pathway, including the alterations from PIK3CA, PTEN, MORT, STK11, TSC1, RPTOR, and NF2; 50% (20 of 40) harbored alterations within cell-cycle–associated genes, including CDKN2A/B, RB1, CCND2/3, CDK4, and CCNE1; 45% (18 of 40) harbored alterations within other tyrosine kinase receptor genes, including EGFR, ERBB2/3, FGFR3/4, KDR, FLT4, PDGFRB, DDR2, and JAK3; and 55% (22 of 40) harbored alterations within TP53-associated genes, including TP53, ATM, and MDM2 (Supplementary Fig. 2).
The most enriched co-occurrence of mutated genes with NTRK fusions were TP53 (38%, 15/40), CDKN2A (23%, 9/40), and ACVR2A (18%, 7/40). CDKN2A/2B homozygous deletion exclusively occurs in soft tissue sarcoma patients with NTRK1 fusions. Alterations in ATM (4 vs. 1), LRP1B (3 vs. 1), and PTEN (2 vs. 0) were more frequently observed in patients with NTRK3 fusions than in those with NTRK1 fusions. We also found a 42-year-old male patient (Patient 16) with stage I retroperitoneal liposarcoma who carried double NTRK3 fusions, such as MORF4L1-NTRK3 and PPFIA2-NTRK3, accompanied by the amplifications of CDK4, IDH2, MDM2, TERT, and TNFSF11.
Three EGFR-activated alterations coupled with NTRK fusions were detected in lung cancer patients, including two non-small cell lung cancer patients and one small cell lung cancer patient. These patients were previously treated with EGFR tyrosine kinase inhibitor (TKI). A 43-year-old female patient diagnosed with advanced non-small cell lung cancer with bone metastasis harbored the EGFR exon 19 deletion and received the treatment of erlotinib, and the mass of the right lung tumor was reduced after about 2 months of treatment. However, four more months later, an enlarged mass on the left lung tumor was observed, and the mutation of EGFR T790M was detected. After receiving the treatment of osimertinib for about 11 months, her disease progressed. Then the mutation of EGFR exon 19 deletion and a gene fusion of TPM3-NTRK1 were detected but without the mutation of EGFR T790M. Besides, a single nucleotide variant (SNV) of TP53 P278A was also detected.
NTRK SNVs and amplifications
The frequency of NTRK amplification in our cohort with solid tumors was approximately 0.8% (N = 86). NTRK amplifications were detected in hepatocellular carcinoma (35/1133, 3.1%), soft tissue sarcoma (12/571, 2.1%), intrahepatic cholangiocarcinoma (8/555, 1.4%), gastric cancer (4/866, 0.5%), and non-small cell lung cancer (4/2039, 0.2%) (Supplementary Fig. 3a). The most frequent NTRK amplification occurred in NTRK1 (92%, 79/86), followed by NTRK3 (7%, 6/86), and NTRK2 (1%, 1/86).
The frequency of NTRK SNVs was higher than that of NTRK fusions and NTRK amplifications. The frequency of NTRK SNVs in our cohort with solid tumors was approximately 3.0% (N = 302). The NTRK SNVs were frequently detected in colorectal cancer (68/1225, 5.6%), non-small cell lung cancer (62/2039, 3.0%), gastric cancer (43/886, 5.0%), hepatocellular carcinoma (24/1133, 2.1%), and small cell lung cancer (13/220, 5.9%) (Supplementary Fig. 3b). The most frequent NTRK SNV occurred in NTRK3 (54.3%, 164/302), followed by NTRK2 (24.5%, 74/302), and NTRK1 (21.1%, 64/302). Variants in the solvent front, the gatekeeper, or the xDFG motif of the kinase domain have been reported as acquired resistant variants to TRK inhibitors such as NTRK1 G595R/F589L/G667C/S, NTRK2 G639R/F633L/G709C, and NTRK3 G696A/G623R/F617L13,14. These variants were not observed in any of the 10,194 combined adult and pediatric tumors.
We also found that NTRK amplifications and NTRK SNVs rarely occurred with NTRK fusions, which were 5.0% (2/40) and 2.5% (1/40), respectively. Furthermore, tumors with NTRK SNVs had higher TMB than those with NTRK amplifications, NTRK fusions, or those without NTRK alteration (P < 0.001, respectively) (Supplementary Fig. 4).
Comparison of immunohistochemistry, DNA-, and RNA-based NGS assays
The NTRK fusions can be detected by multiple platforms, such as FISH, RT-PCR, or NGS, and the overexpression of TRK proteins caused by fusion events can be confirmed by IHC. In our study, 13 NTRK fusion-positive samples detected by DNA-based NGS assay also underwent RNA-based NGS assay, and TRK expression in 9 samples was further confirmed by IHC detection. Results showed that for 12 of 13 patients, NTRK fusions were also identified by RNA-seq (Table 3). For one remaining patient (Patient 11) with a likely fusion of C7orf69-NTRK3, RNA-seq did not confirm the presence of the fusion transcript. However, due to insufficient samples, we failed to conduct further validation by NGS. For an infantile fibrosarcoma patient (patient 2, Table 3) and a colorectal carcinoma patient (patient 39, Table 3), the fusions of ETV6-NTRK3 were detected by both DNA- and RNA-based NGS assays, but the pan-TRK IHC was negative. Consistent with the results of DNA- and RNA-based NGS detection, the expression of NTRK was confirmed by IHC in seven samples (Table 3). Together, our results indicate that NGS detection based on the combination of DNA and RNA can effectively meet clinical NTRK fusion detection.
Clinical response for NTRK fusion-positive patients
In our study, two patients underwent treatment with TKIs, such as larotrecitnib. The first patient (Patient 33) is a 63-year-old Chinese male who was diagnosed with lung adenocarcinoma accompanied by mediastinal lymph node metastasis and double lung metastasis with a rare PRDX1-NTRK1 fusion (Fig. 3a) and an MTOR E2419D mutation. A pan-TRK IHC assay also indicated that this patient was TRK-positive. Subsequently, he was treated with crizotinib at a dose of 85 mg twice a day. One month later, this patient achieved a partial response with a reduction in lung lesions and the disappearance of pleural effusion. After 4 months of crizotinib treatment, he received the combination therapy of pemetrexed and cisplatin for 5 months. Subsequently, he was successfully recruited for an ongoing clinical trial of the selective TRK inhibitor larotrectinib, and the treatment started in March 2019. The patient experienced partial remission after 2 months (Fig. 3b) and had a sustained durable response to larotrectinib until April 2021.
a The PRDX1 exon 5-NTRK1 exon 12 fusion in Integrative genomics viewer (IGV) from the patient (Patient 33) with lung adenocarcinoma. IGV screenshot showed the breakpoints on the intron 5 of PRDX1 gene (left) and on the intron 11 of NTRK1 gene (right) detected by capture-based next-generation sequencing. b CT scans of Patient 33 before and after 2 months of larotrectinib treatment. c The illustration of LMNA-NTRK1 fusion at DNA and RNA levels in Patient 15. d Radiological comparison of abdominopelvic CT scans before and after 2 months of larotrectinib treatment by coronal and transverse planes of Patient 15.
The second patient (Patient 15) is a 42-year-old Chinese female who was diagnosed with advanced spindle cell fibrosarcoma of the thigh in 2009. Over the subsequent 9 years, she underwent multiple lines of treatment without achieving effective control of the disease. In March 2018, an LMNA-NTRK1 fusion involving exons 1 and 2 of LMNA and exons 10–17 of the NTRK1 gene was identified by NGS (Supplementary Fig. 5). The LMNA-NTRK1 fusion detected by DNA-seq and RNA-seq is illustrated in Fig. 3c. She was recruited for an ongoing clinical trial of the selective TRK inhibitor larotrectinib, and the treatment was started in June 2018. After 2 months of treatment, a CT scan revealed stable disease according to RECIST1.1 (Fig. 3d). To our knowledge, this is a rare report on the clinical response to larotrectinib in a Chinese patient with spindle cell fibrosarcoma harboring an LMNA-NTRK1 fusion.
Discussion
Gene fusions involving ALK and ROS1 have been increasingly recognized as clinically actionable alterations. NTRK fusions have been reported to promote tumorigenesis by constitutively activating downstream cell growth and proliferative pathways, resulting in pathways addiction and making them attractive targets of cancer therapy15. In addition, the presence of NTRK fusions in certain cancers, such as non-small cell lung cancer, is associated with poor survival16. Although NTRK fusions are infrequent in solid tumors (typically seen in <1% of patients), both the present data and previous reports demonstrate that NTRK fusions are enriched in certain histologic subsets, including pediatric fibrosarcomas, secretory carcinoma of the breast, and mammary analog secretory carcinoma12,17.
In the present study, 0.4% (40/10,194) Chinese solid tumor patients harbored NTRK fusions, whereas, in a report on a Western cohort, 0.27% of 11,502 solid tumor patients were found to have NTRK fusions18. Of note, the frequency of NTRK1 fusion in non-small cell lung cancer was higher than that from another report in the Chinese population (0.17% vs. 0.073%)19. This is a large-scale study on the distribution of NTRK fusions in Chinese patients with a variety of solid tumors. Consistent with previous studies20,21, our findings also showed a relatively high frequency of NTRK fusions in patients with fibrosarcoma and thyroid tumor. Several NTRK fusions identified in our study have been previously reported as activating and oncogenic fusions involved in cancer cell growth, proliferation, and poor survival4,16,22. Protein domain analyses indicate that these fusions, as well as the 11 rare fusions, contain the TRK tyrosine kinase domain regardless of the 5’ fusion partner.
The fusion of NTRK3 is rare in tumors, and the fusion of NTRK3 with two partner genes in one patient is even rarer. We identified two NTRK3 fusions in a male patient, namely MORF4L1-NTRK3 and PPFIA2-NTRK3. The breakpoint of NTRK3 both occurred in intron 13, while the partner genes were located on chromosome 15 (MORF4L1) and chromosome 12 (PPFIA2), respectively. According to the results of genome alignment, the fusions of MORF4L1-NTRK3 and PPFIA2-NTRK3 can be supported by 255 and 198 pairs of reads, respectively. However, the lack of sufficient samples for further validation is a limitation of this study. Based on the NGS-based genomic detections, we explored the mutational characteristics of 40 patients with NTRK fusions. Our results showed that NTRK fusions co-occur with TP53-associated genes and cell cycle-associated genes. Rosen et al. reported that NTRK fusions were rarely co-mutated with other canonical oncogenes, including EGFR23. Previous reports showed that NTRK fusion might be the acquired resistant variant for the EGFR TKI in lung cancer patients24. In this study, EGFR variants were detected in 10% of patients with NTRK fusions. For one patient (patient 18), the TPM3-NTRK1 fusion was identified after the disease progressed on osimertinib. In this patient, the TPM3-NTRK1 fusion may be a potential mechanism of acquired osimertinib resistance17,25. TRK inhibitors have attracted considerable attention in the past few years due to the dramatic, long-lasting response observed in patients with NTRK fusion who received therapy in early clinical trials22,26. Though multitargeted tyrosine kinase inhibitors such as crizotinib demonstrated efficacy in patients carrying the NTRK1 fusion22,27,28, selective TRK inhibitors revealed superior efficacy. Larotrectinib achieves a 78% objective response rate in tumors harboring NTRK fusion4,6,29,30, and it was approved by the FDA for the treatment of metastatic solid tumors carrying NTRK fusion, regardless of the underlying tumor histology12. Since novel selective inhibitors of constitutively active rearranged proteins have been developed, the ability to detect NTRK fusions will have a significant impact on clinical practice.
Similar to the previous study17, we also identified an association between NTRK fusion and MSI-H. The development of colorectal cancer is often accompanied by the occurrence of MSI-H or chromosomal instability25. This explains the high proportion of colorectal cancer in NTRK fusion-positive patients with MSI-H. MSI-H and NTRK fusion positivity are both biomarkers of pan-cancer. MSI-H is associated with high immune scores and a higher response to immune checkpoint inhibitors31,32, while NTRK has dual functions of promoting nervous system development and carcinogenesis33,34. TRK inhibitors such as larotrectinib have shown good efficacy in patients with NTRK fusions22,26. However, in the presence of two biomarkers simultaneously, the efficacy of monotherapy and combination therapy is intriguing, and further clinical research is needed to confirm.
NGS-based DNA detection can maximize the detection of genomic target site variations, while NGS-based RNA detection can identify target genes at the transcriptome level, which is conducive to increasing the fusion detection rate and discovering unknown fusions35. FISH is well established as the diagnostic gold standard for fusion gene detection36. Ben et al. also showed that the combination of DNA-seq and RNA-seq could effectively enhance the detection of gene fusions37. In our study, 12 of 13 NTRK fusion-positive cases detected by DNA-based NGS were also detected by RNA-based NGS assay. The failure of NTRK fusion detection in one sample at the RNA level was probably caused by the low level of transcription or degradation due to sample handling or by nonsense-mediated decay. This sample was also negative for IHC. One common fusion, ETV6-NTRK3 (Patient 2, Table 3), was detected by both NGS-based RNA-seq and DNA-seq, but the protein was not detected by pan-TRK IHC. This might have been caused by protein degradation, which showed the limitations of this technology. Together, NGS-based DNA-seq and RNA-seq can confirm each other, and simultaneously detecting both is the better strategy for gene fusion detection. Two of our patients with NTRK fusions at the DNA level and known treatment histories received crizotinib or larotrectinib treatment and showed good clinical responses. This implies the advantage of NGS for NTRK fusion detection: It allows numerous genes to be analyzed in a much less labor-intensive manner than that with Sanger sequencing and detects more variant types, such as gene-activating SNVs, in-frame indel, and amplification or rearrangements, which contributes to a more comprehensive clinical landscape and more precision clinical practice.
In conclusion, we identified nearly 0.4% of NTRK fusion events in a large Chinese cohort, described the mutational characterization of NTRK fusion-positive patients, and reported the real cases of patients with NTRK fusion who benefit from larotrectinib. Our results showed that NGS detection, including DNA-based and the combination of DNA-based and RNA-based, can effectively assist clinical NTRK fusion detection and is of great significance for the identification of novel NTRK fusions. This study profiled the prevalence and molecular distribution of NTRK fusions in Chinese solid tumor patients, which supported the application of NGS to clinical oncology practice and guided the use of TRK inhibitors to help patients with such rare genomic alterations improve their clinical performance.
Methods
Patients
This study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of the Shandong Provincial Hospital and the Shanghai Ethics Committee for Clinical Research. All patients provided written informed consent. From 2017 to 2018, a total of 10,194 pathologically diagnosed solid tumor samples, either resected or biopsied, were collected from patients across all of China, including 4222 (41.4%) from East China, 2983 (29.3%) from South China, 966 (9.5%) from Southwest China, 953 (9.3%) from North China, 505 (5.0%) from Central China, 333 (3.3%) from Northwest China, and 232 (2.3%) from Northeast China38. Formalin-fixed paraffin-embedded (FFPE) tumor tissues and matched blood samples were collected to detect genomic alterations.
Next-generation sequencing
All samples were subjected to sequencing at OrigiMed (OrigiMed, Inc., Shanghai), a College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA) certified laboratory (Certificate ID: 99D2159871). Genomic DNA was extracted from 10,194 samples using a DNA Extraction Kit (QIAamp DNA FFPE Tissue Kit) according to the manufacturer’s protocols. And typically, 50–250 ng of double-stranded DNA was fragmented to about 250 bp by sonication. Subsequent library construction using the KAPA Hyper Prep Kit (KAPA Biosystems) for end repair, dA addition, and adapter ligation was performed, followed by PCR amplification and quantified by Qubit assessment.
RNA was successfully extracted from unstained FFPE sections of 356 cases (miRNeasy FFPE Kit, Qiagen) according to the manufacturer’s protocol. A cDNA primer mixture of random hexamer and oligo dT (Thermo Fisher Scientific) was annealed to the template RNA at 70 °C for 5 min. First strand synthesis was performed using M-MLV RT RNase(H-) (Promega) and followed by second strand synthesis (NEB). The cDNA was cleaned up using 1.8× Agencourt RNA Clean XP Beads (Beckman). The entire cDNA product was sheared by sonication (E220, Covaris) to the fragment of about 200 bp before library construction. Adapters were ligated to the libraries (KAPA Hyper Prep Kit, Roche) and quantified by the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific).
A custom hybridization panel with probes against all exons and selected introns (such as NTRK1 introns 3–23, NTRK2 introns 11, 12, and 15, and NTRK3 introns 10 and 12–15) of NTRK1, NTRK2, and NTRK3 that applied to DNA-seq and a custom hybridization panel with probes against all exons of NTRK1, NTRK2, and NTRK3 that applied to RNA-seq were used to capture the targeted sequences followed the protocol of “Hybridization capture of DNA libraries using xGen® Lockdown® Probes and Reagents” (Integrated DNA Technologies). Post-capture libraries were mixed together, denatured and diluted to 1.5–1.8 pM, and subsequently sequenced on Illumina NextSeq 500. Tumor samples were sequenced to a median unique coverage of 1202× and matched normal blood samples were sequenced to a mean unique coverage of 300×.
Bioinformatics pipeline for genomic alteration
Resultant sequences were mapped to the hg19 reference genome with BWA (version 0.7.12). SNVs were identified using MuTect (v1.7); short Insertions/deletions (Indels) were identified using PINDEL (V0.2.5); copy number variations (CNVs) were identified using EXCAVATOR (v2.2, http://sourceforge.net/projects/excavatortool/); TMB was calculated by counting the number of coding SNVs and indels per megabase of the sequence examined; the state of microsatellite stability was determined by candidate MSI markers including 572 identified microsatellite loci, and MSI-H is defined as more than 15% of selected microsatellite loci showing unstable in tumors compared to matched peripheral blood39,40. The in-house developed algorithm was used for DNA fusion detection and is detailed as follows: aligned reads with an abnormal insert size of over 2000 bp or matched to two different chromosomes were collected and used as discordant reads, i.e., paired-end reads that could not be closely mapped to a genome reference, with each read of paired-reads aligned to the same chromosomes or different chromosomes. Next, the discordant reads with a distance less than 500 bp formed clusters that were further assembled by Fermi-lite to identify potential rearrangement breakpoints40,41. The breakpoints were double-confirmed by BLAT, and the resulting chimeric gene candidates were annotated. At least five unique supporting read pairs were necessary for a genomic alteration. For RNA-Seq data, the STAR (v 2.5.3) algorithm was used to locate RNA-Seq readings, and STAR fusion (version 0.8) was used for fusion detection42. Gene fusion or rearrangements were finally assessed by Integrative Genomics Viewer (IGV).
Pan-TRK immunohistochemistry
Briefly, slides were baked at 60 °C, deparaffinized in xylenes, and rehydrated with graded ethanol to distilled water. Antigen retrieval was performed using a Dako EnVision FLEX Target Retrieval Solution, high pH, in a steamer. Cooled slides were stained using an automated IHC staining platform. IHC staining for TRK A, B, and C expression was performed with a pan-TRK monoclonal antibody (mAb) clone EPR17341 (Abcam, Cambridge, MA)9,10. This antibody is reactive to a homologous region of TRKA, TRKB, and TRKC near the C terminus. All other staining was performed primarily with the Dako series reagents (K8002). EnVision FLEX+ wash buffer was used between incubation steps, and slides were counterstained with hematoxylin. Slides were then rinsed in distilled water and subjected to an ethanol dehydration series and xylene changes before coverslipping. Brain tissue was used as a positive control, and colorectal epithelium was used as a negative external control. Specimens were scored positive by pathologists if the specimens exhibited any staining intensity in >5% of tumor cells. Specimens without any visible or faint staining in tumor cells were scored negative.
Statistical analysis
Statistical analysis was conducted using the R Statistical Software package (version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria). Categorical variables are presented as numbers and percentages; medians and percentiles are reported for continuous variables. In multiple-group comparisons, Kruskal–Wallis rank-sum tests, Chi-square tests, or Fisher’s exact tests, with Bonferroni post-hoc comparisons, were used. The threshold for statistical significance was set at P < 0.05. The Circos plot was generated using the online Circos Table Viewer (http://mkweb.bcgsc.ca/tableviewer).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All genomic data used in this study are available online (https://www.cbioportal.org/study/summary?id=pan_origimed _2020). All processed data generated for this study are available from the authors upon request.
Code availability
All codes used in this manuscript are publicly available [http://ftp.origimed.com/gravityproject].
References
Fuse, M. J. et al. Mechanisms of resistance to NTRK inhibitors and therapeutic strategies in NTRK1-rearranged cancers. Mol. Cancer Ther. 16, 2130–2143 (2017).
Jabbar, K. J. et al. Comparison of next-generation sequencing mutation profiling with BRAF and IDH1 mutation-specific immunohistochemistry. Am. J. Surg. Pathol. 39, 454–461 (2015).
Amatu, A., Sartore-Bianchi, A. & Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 1, e000023 (2016).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
Okamura, R. et al. Analysis of NTRK alterations in pan-cancer adult and pediatric malignancies: implications for NTRK-targeted therapeutics. JCO Precis. Oncol. 2018, PO.18.00183 (2018).
Meldolesi, J. Neurotrophin Trk receptors: new targets for cancer therapy. Rev. Physiol. Biochem. Pharmacol. 174, 67–79 (2018).
Scott, L. J. Larotrectinib: first global approval. Drugs 79, 201–206 (2019).
Hsiao, S. J., Zehir, A., Sireci, A. N. & Aisner, D. L. Detection of tumor NTRK gene fusions to identify patients who may benefit from tyrosine kinase (TRK) inhibitor therapy. J. Mol. Diagn. 21, 553–571 (2019).
Rudzinski, E. R. et al. Pan-Trk immunohistochemistry identifies NTRK rearrangements in pediatric mesenchymal tumors. Am. J. Surg. Pathol. 42, 927–935 (2018).
Hechtman, J. F. et al. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am. J. Surg. Pathol. 41, 1547–1551 (2017).
Skalova, A. et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard RT-PCR: report of 4 cases harboring ETV6-X gene fusion. Am. J. Surg. Pathol. 40, 3–13 (2016).
Schram, A. M., Chang, M. T., Jonsson, P. & Drilon, A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 14, 735–748 (2017).
Drilon, A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 30, viii23–viii30 (2019).
Rolfo, C. et al. NTRK1 fusions identified by non-invasive plasma next-generation sequencing (NGS) across 9 cancer types. Br. J. Cancer 126, 514–520 (2022).
Passiglia, F. et al. The potential of neurotrophic tyrosine kinase (NTRK) inhibitors for treating lung cancer. Expert Opin. Investig. Drugs 25, 385–392 (2016).
Okamura, K. et al. Expression of TrkB and BDNF is associated with poor prognosis in non-small cell lung cancer. Lung Cancer 78, 100–106 (2012).
Westphalen, C. B. et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis. Oncol. 5, 69 (2021).
Gatalica, Z., Xiu, J., Swensen, J. & Vranic, S. Molecular characterization of cancers with NTRK gene fusions. Mod. Pathol. 32, 147–153 (2019).
Xia, H. et al. Evidence of NTRK1 fusion as resistance mechanism to EGFR TKI in EGFR+ NSCLC: results from a large-scale survey of NTRK1 fusions in Chinese patients with lung cancer. Clin. Lung Cancer 21, 247–254 (2019).
Siozopoulou, V., Smits, E., De Winne, K., Marcq, E. & Pauwels, P. NTRK fusions in sarcomas: diagnostic challenges and clinical aspects. Diagnostics 11, 478 (2021).
Pekova, B. et al. NTRK fusion genes in thyroid carcinomas: clinicopathological characteristics and their impacts on prognosis. Cancers 13, 1932 (2021).
Vaishnavi, A. et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat. Med. 19, 1469–1472 (2013).
Rosen, E. Y. et al. TRK fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin. Cancer Res. 26, 1624–1632 (2020).
Helman, E. et al. Cell-free DNA next-generation sequencing prediction of response and resistance to third-generation EGFR inhibitor. Clin. Lung Cancer 19, 518–530.e517 (2018).
Sato, K. et al. Fusion kinases identified by genomic analyses of sporadic microsatellite instability-high colorectal cancers. Clin. Cancer Res. 25, 378–389 (2019).
Doebele, R. C. et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov. 5, 1049–1057 (2015).
Wong, V. et al. Evaluation of a congenital infantile fibrosarcoma by comprehensive genomic profiling reveals an LMNA-NTRK1 gene fusion responsive to crizotinib. J. Natl Cancer Inst. 108, djv307 (2016).
Chen, W. et al. Unclassified mesenchymal sarcoma with NTRK1-KHDRBS1 gene fusion: a case report of long-term tumor-free survival with crizotinib treatment. World J. Surg. Oncol. 19, 136 (2021).
Drilon, A. et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 7, 963–972 (2017).
Hong, D. S. et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 21, 531–540 (2020).
Eso, Y., Shimizu, T., Takeda, H., Takai, A. & Marusawa, H. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J. Gastroenterol. 55, 15–26 (2020).
Lizardo, D. Y. et al. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: from bench to bedside. Biochim. Biophys. Acta Rev. Cancer 1874, 188447 (2020).
Arévalo, J. C. & Wu, S. H. Neurotrophin signaling: many exciting surprises. Cell. Mol. Life Sci. 63, 1523–1537 (2006).
Nakagawara, A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 169, 107–114 (2001).
Heyer, E. E. & Blackburn, J. Sequencing strategies for fusion gene detection. Bioessays 42, e2000016 (2020).
Byron, S. A., Van Keuren-Jensen, K. R., Engelthaler, D. M., Carpten, J. D. & Craig, D. W. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat. Rev. Genet. 17, 257–271 (2016).
Beaubier, N. et al. Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget 10, 2384–2396 (2019).
Wu, L. et al. Landscape of somatic alterations in large-scale solid tumors from an Asian population. Nat. Commun. 13, 4264 (2022).
Guo, Y. et al. Genomic alterations of NTRK, POLE, ERBB2, and microsatellite instability status in Chinese patients with colorectal cancer. Oncologist 25, e1671–e1680 (2020).
Cao, J. et al. An accurate and comprehensive clinical sequencing assay for cancer targeted and immunotherapies. Oncologist 24, e1294–e1302 (2019).
Li, H. FermiKit: assembly-based variant calling for Illumina resequencing data. Bioinformatics 31, 3694–3696 (2015).
Haas, B. J. et al. Accuracy assessment of fusion transcript detection via read-mapping and de novo fusion transcript assembly-based methods. Genome Biol. 20, 213 (2019).
Acknowledgements
The authors are grateful to all the study participants, patients, and their family members for their contributions and support.
Author information
Authors and Affiliations
Contributions
K.W. and D.S.W.T. conceived and designed the study. Y.X., W.W., X.D., L.Q., D.S.W.T., and K.W. collected and managed the data. X.S., L.Z., S.C., M.R., N.B., and S.Y. performed the statistical analyses. All authors were involved in writing and reviewing the manuscript and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Y., Shi, X., Wang, W. et al. Prevalence and clinico-genomic characteristics of patients with TRK fusion cancer in China. npj Precis. Onc. 7, 75 (2023). https://doi.org/10.1038/s41698-023-00427-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-023-00427-3
- Springer Nature Limited