Abstract
Dietary intake has an undeniable role in the development and progression as well as the prevention and treatment of cirrhosis. This study was conducted with the aim of investigating the association between dietary inflammatory indices and total mortality in patients with cirrhosis. A total of 166 outpatients with cirrhosis who were diagnosed within the last 6 months were followed up for 48 months in this cohort study. A 168-question valid food frequency questionnaire was used to evaluate dietary intake. Accordingly, the dietary inflammatory index (DII), empirical dietary inflammatory pattern (EDIP) and dietary inflammatory score (DIS) were calculated. Multivariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated through cox proportional hazards regression models for an association of cirrhosis mortality and three dietary inflammatory indices. After full adjustment for confounders, the results showed that mortality risk increased significantly with increasing dietary inflammatory indices. Compared to the first tertile, the risk of mortality due to cirrhosis was associated with 4.8 times increase in the third tertile of DII (HR = 4.8, 95% CI = 1.1–19.8, p trend = 0.029), 3.3 times in the third tertile of EDIP (HR = 3.3, 95% CI = 1.3–8, p trend = 0.004), and 2.2 times increased in the third tertile of DIS (HR = 2.2, 95% CI = 1–4.7, p trend = 0.032). The results of the present study indicated a significant association between dietary inflammatory indices and total mortality among patients with cirrhosis. Additional research is necessary to confirm our findings.
Similar content being viewed by others
Introduction
Cirrhosis is the result of chronic liver disease characterized by scarring of the liver1. Also, cirrhosis is one of the public health problems with high mortality and about one million preventable deaths from cirrhosis are reported worldwide annually2. According to the Global Burden of Disease report in 2017, 1.42% of all deaths in Iran were attributed to cirrhosis and other liver diseases3. The main causes of cirrhosis include infection, hepatitis B and C, metabolic dysfunction-associated steatotic liver disease (MASLD), metabolic syndrome, and alcohol consumption4. Pathologically, all cases of liver cirrhosis share common features such as degeneration and necrosis of hepatocytes, replacement of liver tissue with fibrotic tissue, and loss of liver function5. The commencement and progression of liver fibrosis and cirrhosis involve various cell types, cytokines, and microRNAs5. Excess inflammation can lead to the generation of reactive oxygen and nitrogen species, along with the upregulation of pro-inflammatory cytokine genes5. There is a consensus that oxidative stress and inflammation have a substantial role in the progression of cirrhosis and may eventually lead to hepatocellular carcinoma6.
Diets rich in pro-inflammatory foods stimulate systemic inflammation and increase the level of inflammatory factors in the body. This can increase the risk of chronic diseases and lead to clinical weakness with symptoms such as muscle atrophy, difficulty breathing, chewing or swallowing, as well as loss of the ability to walk7,8. The Dietary Inflammatory Index (DII) has developed as a useful tool to assess the inflammatory potential of the diet9. This index quantifies the inflammatory effects of diet and can help identify dietary patterns that promote inflammation7. In addition to DII, the empirical dietary inflammatory pattern (EDIP) score and dietary inflammation score (DIS) indicate the collective contribution of dietary intake to systemic inflammation10. On the one hand, the correlation of these indices with serum inflammatory biomarkers has been determined, and on the other hand the importance of healthy food choices should be noted, especially because adopting a healthy eating pattern is a vital step in reducing inflammation-related chronic diseases11. These indices are innovative approaches that can provide valuable insights into the role of diet in inflammation and its impact on overall health7. Considering the critical role of diet in the etiology and management of cirrhosis and the possibility of applying dietary modification without side effects and minimal cost, dietary modifications should certainly be considered3.
The association of hepatic fat accumulation with inflammatory markers has been reported previously12. It has also been shown that the prevalence of MASLD is directly related to the intake of foods that have a higher inflammatory index, including simple sugar, red meat, processed foods, saturated fat, and soft drinks13. Increased occurrence of cirrhosis risk factors including MASLD and metabolic-associated fatty liver with consumption of pro-inflammatory foods, western dietary pattern, and higher EDIP and DIS scores have been revealed in various studies14.
Most studies have pointed to systemic inflammation in cirrhosis, and limited studies have addressed the role of diet. Based on the crucial role of diet in the pathogenesis and exacerbation of liver diseases and the fact that inflammation is a potential diet-related mechanism for the development of MASLD and its progression to cirrhosis10,14, diet appears to be an essential moderator of chronic inflammation15. Therefore, this study was conducted with the aim of investigating the association between dietary inflammatory indicators and total mortality in patients with cirrhosis.
Methods and materials
Study population and study design
The present study was based on a cohort design in patients with liver cirrhosis. The diagnosis of cirrhosis was made based on biopsy results. The research was permitted by the ethics committee of the National Nutrition and Food Technology Research Institute (NNFTRI) (IR. SBMU. NNFTRI.1396.186). Moreover, the latest version of the Helsinki Declaration was followed throughout the entire procedure. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines for cohort studies were followed in this study.
A total of 166 adult outpatients with cirrhosis, diagnosed for less than 6 months, were recruited from two teaching hospitals in Tehran, Iran, including Ayatollah Taleqani Hospital and Shariati Hospital, which are affiliated with Shahid Beheshti University of Medical Sciences (SBMU) and Tehran University of Medical Sciences (TUMS), respectively. Patients who had diabetes mellitus, kidney failure, chronic heart disease, malignancy, infectious diseases, pancreatic insufficiency, acquired immunodeficiency syndrome, or were pregnant or breastfeeding were not included in the study. Before enrolment, written informed consent was obtained from all patients.
Participants were enrolled in the study from 2016 to 2018 and followed up annually until 2022. Follow-up was done through annual telephone calls to the participants, during which a questionnaire about death or any medical event was completed.
Dietary assessment
At the beginning of the study, data related to the participants' dietary intake were collected by a skilled dietitian through a face-to-face individual interview with a reliable and valid 168-question food frequency questionnaire (FFQ)16. It should be noted that failure to answer or incomplete answer to more than 10 questions, out of 168 items, was considered a lack of information. After describing the household portion sizes of each food item, the number of times that each food item was consumed in the last year was recorded based on the monthly, weekly, and daily frequency and then converted into grams. Nutrient and energy content calculations were performed using Nutritionist IV software and US Department of Agriculture (USDA) Food Composition Table (FCT). For traditional Iranian foods that were not included in the USDA FCT, the Iranian FCT was used.
Calculation of dietary inflammatory index
The basis of the calculation of the dietary inflammatory index was the study of Shivappa et al.9, in which the calculation of the DII is fully described. In summary, DII was established based on the effect of forty-five different nutritional parameters including macro- and micronutrients and bioactive substances on six inflammatory biomarkers. Foods with potential inflammatory effect were scored as +1 and foods with anti-inflammatory effect as −1. Finally, by summing the DII scores of each parameter, the total DII score for each participant was calculated. Also, EDIP and DIS were determined based on the instructions provided in previous studies17,18. In the calculation of EDIP, three of the 18 food groups were excluded, which included alcoholic drinks (due to religious considerations wine and beer consumption is not common in Iran and may be underreported) and low-energy beverages due to lack of access in Iran. The daily consumption of each of the 15 food groups was multiplied by the proposed coefficients and finally EDIP was calculated by summing up all the weighted values. To reduce the magnitude of the score, the final score was divided by 1000. To calculate the DIS score, the food groups were first standardized (with a mean of zero and a standard deviation of 1) and then added together. Since no information was available on the intake of supplements, instead of 19 food groups, 18 food groups were included in the DIS score calculation.
Data collection
Information regarding baseline characteristics, anthropometric measurements, alcohol consumption (> 20 g/d), smoking (at least one cigarette per day), in addition to medical history was collected via face-to-face interviews by a trained interviewer. It should be noted that since alcohol consumption is a taboo in our country, the information provided by patients may not be very accurate. Also, none of the patients were former smokers. Body weight was measured with a digital scale and rounded to the nearest 100 g. Height was measured with a portable non-stretch meter and rounded to the nearest 0.5 cm. Then, BMI was determined via weight (in kilograms) divided by the square of height (in meters).
To estimate the nutritional status of patients, subjective global assessment (SGA) was determined and accordingly patients were categorized into three groups: well nourished (A), moderately malnourished (B), and severe malnourished (C)19. The Model for end-stage liver disease (MELD), and Child–Pugh scores were used to evaluate the prognosis and severity of cirrhosis using established formulas20.
Statistical analysis
The statistically significant level was set at P values < 0.05. According to dietary inflammatory indices, patients were divided into three groups to best fit the distribution of the data, simplify interpretation and perform an inter-tertile comparison without extreme differences in sample size between the groups. One-way analysis of variance (ANOVA) for continuous variables and a Chi-square (χ2) for categorical variables were used to compare baseline characteristics of participants among tertiles of dietary inflammatory index. Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated through Cox proportional hazards regression models for cirrhosis mortality associated with tertiles of dietary inflammatory indices. The P-trend was determined using the median of each tertile. The proportional hazards assumption was satisfied based on a Schoenfeld residual plot.
The selection of confounding factors was based on prior knowledge, existing literature and clinical considerations. The potential confounders were included in a subsequent sequence, as follows: Model 1 was adjusted for age and sex; Model 2 was further adjusted for energy intake, BMI, smoking (at least one cigarette a day), and using alcohol (intake of > 20 g/d); and Model 3 was additionally adjusted for etiology (virus, autoimmune, other), MELD score, SGA, and Child–Pugh classification. The person-years of follow-up were considered based on the date of participation until censoring on April 30, 2022, or lost to follow-up or the date of death.
Ethics approval and consent to participate
National nutrition and Food Technology Research Institute (NNFTRI) ethics committee approved the study protocol (IR. SBMU. NNFTRI.1396.186) in accordance with the Declaration of Helsinki. All participants provided written informed consent and were informed about the study.
Results
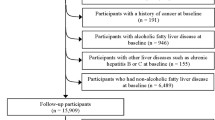
The characteristics of the participants can be seen in previously published paper of the same project4. Among 166 patients who entered the study, 45 patients were excluded because of cancer diagnosis in the first year (n = 2), death due to other causes (one breast cancer, one accident, two COVID-19), lost to follow-up (n = 2), missing or incomplete information on lifestyle or dietary intake (n = 27), extreme energy intake (< 500 or > 5000 kcal/day) (n = 6), and extreme body mass index (BMI) (< 15 or > 50 kg/m2) (n = 4). Therefore, the analyses were done with 121 patients, including 83 men and 38 women (Fig. 1). Patients were divided into tertiles based on DII. As shown in Table 1, there is no significant difference between the three tertiles in terms of the baseline characteristics of the patients, including age, sex, etiology of cirrhosis, anthropometric measures, disease severity, etc. During the follow-up period, 43 deaths were recorded (7 women and 36 men), which were respectively attributed to liver failure (47%), cardiovascular diseases (40%), cancer (3%) and other causes (10%). The cause of death of the patients was determined based on their death certificate.
Table 2 displays the intake of food groups by the participants. Intake of poultry, whole grains, nuts, legumes, fruits, vegetables and dairy products does not show significant differences between DII tertiles. While with increasing DII score, intake of fish decreases and intake of red and processed meat, total meat, refined grains and total grains increases significantly. Schoenfeld residual analysis was performed in all models, and the proportional hazard assumption was not violated. Table 3 describes the risk of total mortality in patients with cirrhosis based on different indices of dietary inflammation. The number of deaths increases significantly with the increase in the scores of all three inflammatory indices. Diet-induced inflammation was associated with an increased propensity for mortality, so higher scores of DII (HRT2 vs T1 = 1.27; 95% CI 0.53–3.1 and HRT3 vs T1 = 2.3; 95% CI 1–4.9; P for trend = 0.031), EDIP (HRT2 vs T1 = 3.6; 95% CI 0.66–19.7 and HRT3 vs T1 = 5.3; 95% CI 1–30; P for trend = 0.053) and DIS (HRT2 vs T1 = 1; 95% CI 0.3–3.7 and HRT3 vs T1 = 2.7; 95% CI 0.9–7.8; P for trend = 0.047) resulted in increased risk of mortality after adjusting the results for age and sex.
Additionally, adjustment for energy intake, BMI, smoking and alcohol yielded similar results for DII and EDIP, but eliminated the significance of the association between mortality and DIS (HRT2 vs T1 = 1.2; 95% CI 0.4–2.7 and HRT3 vs T1 = 2; 95% CI 0.9–4.4; P for trend = 0.066).
Also, further adjustment for other confounding variables including etiology, SGA, MELD and child scores considerably strengthened the initially observed patterns regarding DII (HRT3 vs T1 = 4.8; 95% CI 1.1–19.8; P for trend = 0.029), EDIP (HRT3 vs T1 = 3.3; 95% CI 1.3–8; P for trend = 0.004) and DIS (HRT3 vs T1 = 2.2; 95% CI 1–4.7; P for trend = 0.032).
Furthermore, the 4-year survival rate among patients across tertiles of DII, EDIP and DIS are displayed in Fig. 2a–c, respectively, after adjustment for all confounders. With increasing all three dietary inflammation scores, the survival rate showed a significant decrease.
Kaplan–Meier survival curve for death among cirrhotic patients stratified by tertiles of DII (a), EDIP (b), DIS (c), adjusted for age, sex, energy intake, BMI, smoking and alcohol, etiology, SGA, MELD and Child–Pugh. (a) The 4-year survival rate among patients across tertiles was 40%, 42%, 35%, respectively (log-rank test for homogeneity, P = 0.022). (b) The 4-year survival rate among patients across tertiles was 42%, 40%, 34%, respectively (log-rank test for homogeneity, P = 0.007). (c) The 4-year survival rate among patients across tertiles was 41%, 40%, 33%, respectively (log-rank test for homogeneity, P = 0.017). BMI body mass index, MELD model for end-stage liver disease, SGA subjective global assessment, DII dietary inflammatory index, EDIP empirical dietary inflammatory pattern, DIS dietary inflammatory score.
Discussion
The current cohort study was designed with the hypothesis of an association between diet-induced inflammation and the risk of mortality in patients with cirrhosis. The obtained results confirmed the hypothesis of the study and showed that the risk of mortality increases significantly with the increase in the score of dietary inflammation indices including DII, EDIP and DIS in patients with cirrhosis.
Earlier, the findings of previous studies had confirmed the exacerbation of liver diseases due to inflammation. It has been publicized that increased inflammatory mediators can trigger a cascade of reactions such as lipotoxicity, hepatocellular death, liver inflammation, fibrosis, and pathological angiogenesis21. Since systemic inflammation is considered an important factor in the pathophysiology of cirrhosis22, any extrinsic element that affects the development or exacerbation of inflammation, such as following a pro-inflammatory diet, can increase the likelihood of developing liver diseases such as fatty liver, or increase its progress10. The findings of the present study were also in agreement with previous findings and showed that a higher scores of DII, EDIP and DIS, which indicates a diet rich in pro-inflammatory components10, were associated with an increased mortality in patients with cirrhosis. In a retrospective cohort study with over 2,085,947 person-years of follow-up, it was shown that following a diet high in pro-inflammatory foods significantly increase the risk of developing MASLD and cirrhosis. This association remained significant even after adjusting for potential risk factors14. In another study with a case–control design, it was shown that adherence to a healthy dietary pattern has an inverse relationship with liver fibrosis23, while the Western dietary pattern yielded a positive relationship with liver fibrosis24.
One of the important causes of death in patients with cirrhosis is cardiovascular events. In this regard, various epidemiological studies have already shown that a higher EDIP or DIS score may be associated with a higher probability of developing cardiometabolic complications25. Also, adherence to pro-inflammatory diets was associated with an increased occurrence of risk factors including diabetes and metabolic syndrome (which are considered as the most common cause of cryptogenic cirrhosis26), dyslipidemia27, and central obesity10,27. In agreement, an increased risk of metabolic-related fatty liver with higher DIS scores was demonstrated in a nested case–control study28. In another cohort study, Zhong et al. proposed that adopting an anti-inflammatory diet, characterized by lower DII scores, may effectively reduce the risk of developing primary liver cancer and its mortality29.
Examining the dietary pattern of patients with cirrhosis indicates less consumption of legumes, vegetable oils and sugar-free drinks and higher consumption of soft drinks, sauces and desserts30. This dietary pattern is consistent with higher scores of dietary inflammatory indices found in this study. Higher scores of dietary inflammatory indices indicate lower consumption of antioxidant vitamins, minerals, and phenolic compounds and higher consumption of foods rich in saturated fatty acids, which may further contribute to liver metabolic abnormalities through mechanisms such as oxidative stress and increased visceral obesity10.
One of the notable strengths of this cohort study is that the association between dietary inflammatory markers and the risk of mortality from cirrhosis was examined for the first time. The prospective design with a 4-year follow-up time is another strength. In addition, various confounding factors were considered in the statistical analysis as much as possible.
However, some limitations are also noted that should be considered in the future researches. The relatively small number of participants could limit the accuracy of the observed associations; therefore, caution should be exercised when interpreting the results. Another limitation was the use of FFQs, which may be prone to recall bias and participants may over- or under-report their dietary intake. In addition, about 27% of those enrolled were not included in the analysis (mainly excluded due to the lack of information that was caused by unwillingness to participate in the study), which could introduce bias. Unfortunately, information about the education, socioeconomic status and other demographic features of the patients was not collected, which could be confusing factors in these results. Furthermore, as in many epidemiological studies, there is the possibility of residual, unmeasured confounders, and causal relationships remain unknown.
What can be concluded from the findings of this study is that following a pro-inflammatory diet, characterized by higher DII, DIS and EDIP scores, significantly increases the risk of total mortality in patients with cirrhosis. Nevertheless, further research is needed to confirm these findings and to achieve a better understanding of the hypothesized mechanisms involved.
Data availability
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
References
Pashayee-Khamene, F. et al. Malnutrition and its association with the mortality in liver cirrhosis; A prospective nutritional assessment in two referral centers in Iran. Clin. Nutr. ESPEN 54, 453–458 (2023).
Pashayee-Khamene, F. et al. Dietary protein sources and disease severity, malnutrition and anthropometric measurements in cirrhotic patients. Gastroenterol. Hepatol. From Bed to Bench 12(2), 143 (2019).
Hariri, Z. et al. Dietary fiber intake and mortality among survivors of liver cirrhosis: A prospective cohort study. Heliyon 9(6), e16170 (2023).
Daftari, G. et al. Dietary protein intake and mortality among survivors of liver cirrhosis: A prospective cohort study. BMC Gastroenterol. 23(1), 227 (2023).
Pomacu, M. M. et al. Interrelation of inflammation and oxidative stress in liver cirrhosis. Exp. Ther. Med. 21(6), 1–9 (2021).
Li, S., Hong, M., Tan, H.-Y., Wang, N. & Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid. Med. Cell. Longev. 2016, 4234061 (2016).
Shi, L. Association of energy-adjusted dietary inflammatory index and frailty in older adults with nonalcoholic fatty liver disease. Exp. Gerontol. 182, 112296 (2023).
Londhe, P. & Guttridge, D. C. Inflammation induced loss of skeletal muscle. Bone 80, 131–142 (2015).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R. & Hébert, J. R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17(8), 1689–1696 (2014).
Farhadnejad, H. et al. The association between dietary inflammation scores and non-alcoholic fatty liver diseases in Iranian adults. BMC Gastroenterol. 22(1), 1–9 (2022).
da Silva, A. et al. Pro-inflammatory diet is associated with a high number of cardiovascular events and ultra-processed foods consumption in patients in secondary care. Public Health Nutr. 24(11), 3331–3340 (2021).
Tyrovolas, S. et al. The anti-inflammatory potential of diet and nonalcoholic fatty liver disease: The ATTICA study. Ther. Adv. Gastroenterol. 12, 1756284819858039 (2019).
Valibeygi, A. et al. Dietary inflammatory index (DII) is correlated with the incidence of non-alcoholic fatty liver disease (NAFLD): Fasa PERSIAN cohort study. BMC Nutr. 9(1), 84 (2023).
Ibrahim, M. K. et al. The empirical dietary inflammatory pattern score and the risk of nonalcoholic fatty liver disease and cirrhosis. Hepatol. Commun. 7(10), e0263 (2023).
Phillips, C. M. et al. Dietary inflammatory index and non-communicable disease risk: A narrative review. Nutrients 11(8), 1873 (2019).
Mirmiran, P., Esfahani, F. H., Mehrabi, Y., Hedayati, M. & Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 13(5), 654–662 (2010).
Byrd, D. A. et al. Development and validation of novel dietary and lifestyle inflammation scores. J. Nutr. 149(12), 2206–2218 (2019).
Tabung, F. K. et al. Development and validation of an empirical dietary inflammatory index. J. Nutr. 146(8), 1560–1570 (2016).
Detsky, A. S. et al. What is subjective global assessment of nutritional status?. J. Parenter. Enteral Nutr. 11(1), 8–13 (1987).
Malinchoc, M. et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 31(4), 864–871 (2000).
Luo, Y. & Lin, H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun. Inflamm. Dis. 9(1), 59–73 (2021).
Dirchwolf, M. & Ruf, A. E. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis. World J. Hepatol. 7(16), 1974 (2015).
Suk, K. T. & Kim, D. J. Staging of liver fibrosis or cirrhosis: The role of hepatic venous pressure gradient measurement. World J. Hepatol. 7(3), 607 (2015).
Soleimani, D. et al. Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 12, 315–324 (2019).
Marchesini, G., Forlani, G. & Bugianesi, E. Is liver disease a threat to patients with metabolic disorders?. Ann. Med. 37(5), 333–346 (2005).
García-Compeán, D. et al. The prevalence and clinical characteristics of glucose metabolism disorders in patients with liver cirrhosis. A prospective study. Ann. Hepatol. 11(2), 240–248 (2012).
Soltani, S., Moslehi, N., Hosseini-Esfahani, F. & Vafa, M. The association between empirical dietary inflammatory pattern and metabolic phenotypes in overweight/obese adults. Int. J. Endocrinol. Metab. 16(2), e60048 (2018).
Taheri, E. et al. Dietary and lifestyle inflammation scores are inversely associated with metabolic-associated fatty liver disease among Iranian adults: A nested case–control study. J. Nutr. 152(2), 559–567 (2022).
Zhong, G. C. et al. Dietary inflammatory index and incidence of and death from primary liver cancer: A prospective study of 103,902 American adults. Int. J. Cancer 147(4), 1050–1058 (2020).
Buscail, C. et al. Eating patterns in patients with compensated cirrhosis: A case–control study. Nutrients 10(1), 60 (2018).
Author information
Authors and Affiliations
Contributions
Conceptualization, NK and ZH; Formal analysis, ZY; Methodology, MS, FPK, BH, SA and SK; Project administration, ZY and AH; Writing—original draft, NK, AH and ZY; Writing—review and editing, ZY and AH. All authors read and approved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khodadadi, N., Hekmatdoost, A., Pashayee-Khamene, F. et al. The association of dietary inflammatory indices and mortality in patients with cirrhosis: a cohort based study. Sci Rep 14, 21472 (2024). https://doi.org/10.1038/s41598-024-72485-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72485-x
- Springer Nature Limited